- Record: found
- Abstract: found
- Article: found
COVID-19: viral–host interactome analyzed by network based-approach model to study pathogenesis of SARS-CoV-2 infection
Abstract
Background
Epidemiological, virological and pathogenetic characteristics of SARS-CoV-2 infection are under evaluation. A better understanding of the pathophysiology associated with COVID-19 is crucial to improve treatment modalities and to develop effective prevention strategies. Transcriptomic and proteomic data on the host response against SARS-CoV-2 still have anecdotic character; currently available data from other coronavirus infections are therefore a key source of information.
Methods
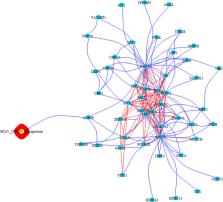
We investigated selected molecular aspects of three human coronavirus (HCoV) infections, namely SARS-CoV, MERS-CoV and HCoV-229E, through a network based-approach. A functional analysis of HCoV–host interactome was carried out in order to provide a theoretic host–pathogen interaction model for HCoV infections and in order to translate the results in prediction for SARS-CoV-2 pathogenesis. The 3D model of S-glycoprotein of SARS-CoV-2 was compared to the structure of the corresponding SARS-CoV, HCoV-229E and MERS-CoV S-glycoprotein. SARS-CoV, MERS-CoV, HCoV-229E and the host interactome were inferred through published protein–protein interactions (PPI) as well as gene co-expression, triggered by HCoV S-glycoprotein in host cells.
Results
Although the amino acid sequences of the S-glycoprotein were found to be different between the various HCoV, the structures showed high similarity, but the best 3D structural overlap shared by SARS-CoV and SARS-CoV-2, consistent with the shared ACE2 predicted receptor. The host interactome, linked to the S-glycoprotein of SARS-CoV and MERS-CoV, mainly highlighted innate immunity pathway components, such as Toll Like receptors, cytokines and chemokines.
Conclusions
In this paper, we developed a network-based model with the aim to define molecular aspects of pathogenic phenotypes in HCoV infections. The resulting pattern may facilitate the process of structure-guided pharmaceutical and diagnostic research with the prospect to identify potential new biological targets.
Related collections
Most cited references52
- Record: found
- Abstract: found
- Article: not found
A Novel Coronavirus from Patients with Pneumonia in China, 2019
- Record: found
- Abstract: found
- Article: found
A pneumonia outbreak associated with a new coronavirus of probable bat origin
- Record: found
- Abstract: found
- Article: not found