- Record: found
- Abstract: found
- Article: found
HPV vaccination in a context of public mistrust and uncertainty: a systematic literature review of determinants of HPV vaccine hesitancy in Europe

Read this article at
ABSTRACT
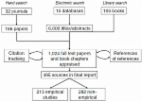
Europe is increasingly described as the region in the world with the least confidence in vaccination, and particularly in the safety of vaccines. The aim of this systematic literature review was to gather and summarise all peer-reviewed and grey literature published about determinants of Human Papillomavirus (HPV) vaccine hesitancy in Europe. Ten thematic categories were identified across the 103 articles which were included in the review. Participants from European studies most commonly reported issues with the quantity and quality of information available about HPV vaccination; followed by concerns about potential side effects of the vaccine; and mistrust of health authorities, healthcare workers, and new vaccines. Comparative analyses indicated that confidence determinants differed by country and population groups. This evidence supports the need to develop context-specific interventions to improve confidence in HPV vaccination and design community engagement strategies aiming to build public trust.
Related collections
Most cited references102

- Record: found
- Abstract: found
- Article: found
The State of Vaccine Confidence 2016: Global Insights Through a 67-Country Survey
- Record: found
- Abstract: found
- Article: not found
Strategies intended to address vaccine hesitancy: Review of published reviews.
- Record: found
- Abstract: found
- Article: found