- Record: found
- Abstract: found
- Article: found
Checkpoint inhibitors as immunotherapy for fungal infections: Promises, challenges, and unanswered questions
Read this article at
Abstract
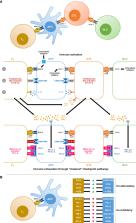
Opportunistic fungal infections have high mortality in patients with severe immune dysfunction. Growing evidence suggests that the immune environment of invasive fungal infections and cancers share common features of immune cell exhaustion through activation of immune checkpoint pathways. This observation gave rise to several preclinical studies and clinical case reports describing blockade of the Programmed Cell Death Protein 1 and Cytotoxic T-Lymphocyte Antigen 4 immune checkpoint pathways as an adjunct immune enhancement strategy to treat opportunistic fungal infections. The first part of this review summarizes the emerging evidence for contributions of checkpoint pathways to the immunopathology of fungal sepsis, opportunistic mold infections, and dimorphic fungal infections. We then review the potential merits of immune checkpoint inhibitors (ICIs) as an antifungal immunotherapy, including the incomplete knowledge of the mechanisms involved in both immuno-protective effects and toxicities. In the second part of this review, we discuss the limitations of the current evidence and the many unknowns about ICIs as an antifungal immune enhancement strategy. Based on these gaps of knowledge and lessons learned from cancer immunology studies, we outline a research agenda to determine a “sweet spot” for ICIs in medical mycology. We specifically discuss the importance of more nuanced animal models, the need to study ICI-based combination therapy, potential ICI resistance, the role of the immune microenvironment, and the impact of ICIs given as part of oncological therapies on the natural immunity to various pathogenic fungi.
Related collections
Most cited references132

- Record: found
- Abstract: found
- Article: found
CTLA-4 and PD-1 Pathways
- Record: found
- Abstract: found
- Article: not found
The diverse functions of the PD1 inhibitory pathway
- Record: found
- Abstract: found
- Article: not found