- Record: found
- Abstract: found
- Article: found
Anaphase Chromosomes in Crane-Fly Spermatocytes Treated With Taxol (Paclitaxel) Accelerate When Their Kinetochore Microtubules Are Cut: Evidence for Spindle Matrix Involvement With Spindle Forces
Read this article at
Abstract
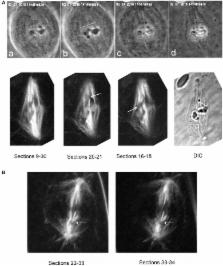
Various experiments have indicated that anaphase chromosomes continue to move after their kinetochore microtubules are severed. The chromosomes move poleward at an accelerated rate after the microtubules are cut but they slow down 1–3 min later and move poleward at near the original speed. There are two published interpretations of chromosome movements with severed kinetochore microtubules. One interpretation is that dynein relocates to the severed microtubule ends and propels them poleward by pushing against non-kinetochore microtubules. The other interpretation is that components of a putative “spindle matrix” normally push kinetochore microtubules poleward and continue to do so after the microtubules are severed from the pole. In this study we distinguish between these interpretations by treating cells with taxol. Taxol eliminates microtubule dynamics, alters spindle microtubule arrangements, and inhibits dynein motor activity in vivo. If the dynein interpretation is correct, taxol should interfere with chromosome movements after kinetochore microtubules are severed because it alters the arrangements of spindle microtubules and because it blocks dynein activity. If the “spindle matrix” interpretation is correct, on the other hand, taxol should not interfere with the accelerated movements. Our results support the spindle matrix interpretation: anaphase chromosomes in taxol-treated crane-fly spermatocytes accelerated after their kinetochore microtubules were severed.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Taxol suppresses dynamics of individual microtubules in living human tumor cells.
- Record: found
- Abstract: found
- Article: not found
Insights into the mechanism of microtubule stabilization by Taxol.
- Record: found
- Abstract: found
- Article: not found