- Record: found
- Abstract: found
- Article: found
Lactobacillus gasseri SBT2055 Induces TGF-β Expression in Dendritic Cells and Activates TLR2 Signal to Produce IgA in the Small Intestine
Read this article at
Abstract
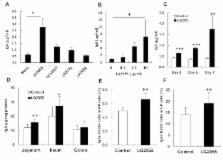
Probiotic bacteria provide benefits in enhancing host immune responses and protecting against infection. Induction of IgA production by oral administration of probiotic bacteria in the intestine has been considered to be one reason for this beneficial effect, but the mechanisms of the effect are poorly understood. Lactobacillus gasseri SBT2055 (LG2055) is a probiotic bacterium with properties such as bile tolerance, ability to improve the intestinal environment, and it has preventive effects related to abdominal adiposity. In this study, we have found that oral administration of LG2055 induced IgA production and increased the rate of IgA + cell population in Peyer's patch and in the lamina propria of the mouse small intestine. The LG2055 markedly increased the amount of IgA in a co-culture of B cells and bone marrow derived dendritic cells (BMDC), and TLR2 signal is critical for it. In addition, it is demonstrated that LG2055 stimulates BMDC to promote the production of TGF-β, BAFF, IL-6, and IL-10, all critical for IgA production from B cells. Combined stimulation of B cells with BAFF and LG2055 enhanced the induction of IgA production. Further, TGF-β signal was shown to be critical for LG2055-induced IgA production in the B cell and BMDC co-culture system, but TGF-β did not induce IgA production in a culture of only B cells stimulated with LG2055. Furthermore, TGF-β was critical for the production of BAFF, IL-6, IL-10, and TGF-β itself from LG2055-stimulated BMDC. These results demonstrate that TGF-β was produced by BMDC stimulated with LG2055 and it has an autocrine/paracrine function essential for BMDC to induce the production of BAFF, IL-6, and IL-10.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells.
- Record: found
- Abstract: found
- Article: not found
Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial.
- Record: found
- Abstract: found
- Article: not found