- Record: found
- Abstract: found
- Article: found
New-onset or flare-up of bullous pemphigoid associated with COVID-19 vaccines: a systematic review of case report and case series studies
Read this article at
Abstract
Background
Numerous cutaneous manifestations have been associated with the Coronavirus Disease 2019 (COVID-19) outbreak and vaccination, but new-onset bullous pemphigoid (BP) or flaring up of pre-existing BP is a rare side effect of COVID-19 vaccines that has been mentioned to a lesser extent in the literature. Therefore, we aimed to conduct a systematic review focused on the association between the new- onset or flare-up of BP and the COVID-19 vaccination.
Method
A comprehensive literature search was conducted using PubMed (MEDLINE), Scopus, and the Web of Science databases up to 11 March 2023. The search aimed to identify English-language studies reporting new-onset or flare-ups of BP as a potential side effect of the COVID-19 vaccination. The search terms included bullous pemphigoid and COVID-19 vaccination-related MeSH terms.
Results
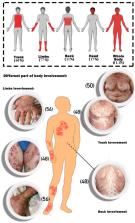
The systematic review of 40 articles investigating the incidence of BP in individuals who received various COVID-19 vaccines revealed pertinent findings. Among the 54 patients with new-onset BP, the median age was 72.42 years, and most were men (64%). Conversely, the median age of the 17 patients experiencing a flare-up of BP was 73.35 years, with a higher proportion of women (53%). Regarding vaccination types, a significant number of patients (56%) developed new-onset BP after receiving the BNT162b2 vaccine (Pfizer-BioNTech).
Conclusion
This study indicates a potential association between COVID-19 vaccinations, particularly mRNA vaccines, and the occurrence of BP. It suggests that this rare autoimmune disorder may be triggered as an adverse event following the COVID-19 vaccination. However, it is important to note that the majority of BP patients in our study were unaffected by the COVID-19 vaccine, and even those who experienced worsening of their conditions were managed without significant consequences. These findings provide additional evidence supporting the safety of COVID-19 vaccines. Physicians should be mindful of this uncommon adverse event and encourage patients to complete their planned vaccination schedules.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
- Record: found
- Abstract: found
- Article: not found
The PRISMA 2020 statement: An updated guideline for reporting systematic reviews
- Record: found
- Abstract: found
- Article: not found