- Record: found
- Abstract: found
- Article: not found
Engineering the Morphology and Particle Size of High Energetic Compounds Using Drop-by-Drop and Drop-to-Drop Solvent–Antisolvent Interaction Methods
Read this article at
Abstract

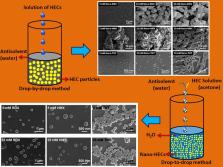
Morphology-controlled precipitation of three powerful organic high energetic compounds (HECs) viz. cyclotrimethylenetrinitramine (RDX), octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX), and 2-methyl-1,3,5-trinitrobenzene (TNT) was achieved by two different processes, namely, drop-by-drop (DBD) and drop-to-drop (DTD) solvent–antisolvent interaction methods. Effect of different experimental parameters on the mean size and morphology of the prepared submicron-sized particles of HECs was investigated thoroughly. The DBD method favors the formation of nanosized particles of RDX and TNT at lower concentrations (5 mM). However, a significant increase in the mean particle size occurred at higher concentrations (25 and 50 mM). Formation of facetted crystals of RDX, HMX, and nanorods of TNT was observed at higher concentrations because of the interaction of crystal facets with the antisolvent. Relatively, smaller sized, spherical particles of RDX and HMX could be prepared through the DTD method even at higher concentrations (25 mM). The DTD method is a continuous process and hence is a facile method for industrial applications. X-ray diffraction and Fourier transform infrared spectroscopy studies revealed that RDX, HMX and TNT were precipitated in their most stable polymorphic forms α, β, and monoclinic, respectively. Differential scanning calorimetry showed that the thermal response of the nano-HECs was similar to the respective raw-HECs. A slight decrease in crystallinity and the melting point was observed because of the decrease in the mean particle size.
Related collections
Most cited references68
- Record: found
- Abstract: not found
- Article: not found
Liquid antisolvent precipitation and stabilization of nanoparticles of poorly water soluble drugs in aqueous suspensions: Recent developments and future perspective
- Record: found
- Abstract: not found
- Article: not found
CL-20 performance exceeds that of HMX and its sensitivity is moderate
- Record: found
- Abstract: not found
- Article: not found
