- Record: found
- Abstract: found
- Article: found
Fibroblast–myocyte electrotonic coupling: Does it occur in native cardiac tissue? ☆
Read this article at
Abstract
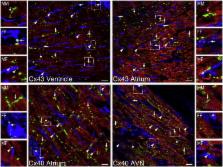
Heterocellular electrotonic coupling between cardiac myocytes and non-excitable connective tissue cells has been a long-established and well-researched fact in vitro. Whether or not such coupling exists in vivo has been a matter of considerable debate. This paper reviews the development of experimental insight and conceptual views on this topic, describes evidence in favour of and against the presence of such coupling in native myocardium, and identifies directions for further study needed to resolve the riddle, perhaps less so in terms of principal presence which has been demonstrated, but undoubtedly in terms of extent, regulation, patho-physiological context, and actual relevance of cardiac myocyte–non-myocyte coupling in vivo. This article is part of a Special Issue entitled "Myocyte-Fibroblast Signalling in Myocardium."
Highlights
-
•
Electrical coupling of cardiomyocytes and fibroblasts is well-established in vitro
-
•
Whether such hetero-cellular coupling exists in vivo has been a matter of debate
-
•
We review the development of experimental and conceptual insight into the topic
-
•
Conclusion 1: hetero-cellular coupling in heart tissue has been shown in principle
-
•
Conclusion 2: extent, regulation, context, and relevance remain to be established
Related collections
Most cited references135
- Record: found
- Abstract: found
- Article: not found
Myofibroblast-mediated mechanisms of pathological remodelling of the heart.
- Record: found
- Abstract: found
- Article: not found
Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation.
- Record: found
- Abstract: found
- Article: not found