- Record: found
- Abstract: found
- Article: not found
Bacterial Plasminogen Receptors: Mediators of a Multifaceted Relationship
review-article
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
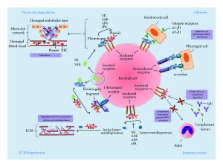
Multiple species of bacteria are able to sequester the host zymogen plasminogen to the cell surface. Once localised to the bacterial surface, plasminogen can act as a cofactor in adhesion, or, following activation to plasmin, provide a source of potent proteolytic activity. Numerous bacterial plasminogen receptors have been identified, and the mechanisms by which they interact with plasminogen are diverse. Here we provide an overview of bacterial plasminogen receptors and discuss the diverse role bacterial plasminogen acquisition plays in the relationship between bacteria and the host.
Related collections
Most cited references158
- Record: found
- Abstract: found
- Article: not found
The urokinase-type plasminogen activator system in cancer metastasis: a review.
P Andreasen, L Kjøller, L. Christensen … (1997)
- Record: found
- Abstract: found
- Article: not found
alpha-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface.
S Hammerschmidt, G. Singh Chhatwal, M Rohde … (2001)
- Record: found
- Abstract: found
- Article: not found
Plasminogen is a critical host pathogenicity factor for group A streptococcal infection.
Hongmin Sun, Ulrika Ringdahl, Jonathon W Homeister … (2004)
