- Record: found
- Abstract: found
- Article: not found
Therapeutic Efficacy of the Small Molecule GS-5734 against Ebola Virus in Rhesus Monkeys
Read this article at
Summary
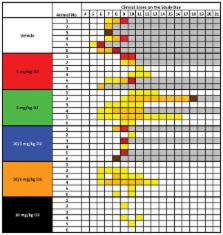
The most recent Ebola virus outbreak in West Africa – unprecedented in the number of cases and fatalities, geographic distribution, and number of nations affected – highlights the need for safe, effective, and readily available antiviral agents for treatment and prevention of acute Ebola virus (EBOV) disease (EVD) or sequelae 1 . No antiviral therapeutics have yet received regulatory approval or demonstrated clinical efficacy. Here we describe the discovery of a novel anti-EBOV small molecule antiviral, GS-5734, a monophosphoramidate prodrug of an adenosine analog. GS-5734 exhibits antiviral activity against multiple variants of EBOV in cell-based assays. The pharmacologically active nucleoside triphosphate (NTP) is efficiently formed in multiple human cell types incubated with GS-5734 in vitro, and the NTP acts as an alternate substrate and RNA-chain terminator in primer-extension assays utilizing a surrogate respiratory syncytial virus RNA polymerase. Intravenous administration of GS-5734 to nonhuman primates resulted in persistent NTP levels in peripheral blood mononuclear cells (half-life = 14 h) and distribution to sanctuary sites for viral replication including testes, eye, and brain. In a rhesus monkey model of EVD, once daily intravenous administration of 10 mg/kg GS-5734 for 12 days resulted in profound suppression of EBOV replication and protected 100% of EBOV-infected animals against lethal disease, ameliorating clinical disease signs and pathophysiological markers, even when treatments were initiated three days after virus exposure when systemic viral RNA was detected in two of six treated animals. These results provide the first substantive, post-exposure protection by a small-molecule antiviral compound against EBOV in nonhuman primates. The broad-spectrum antiviral activity of GS-5734 in vitro against other pathogenic RNA viruses – including filoviruses, arenaviruses, and coronaviruses – suggests the potential for expanded indications. GS-5734 is amenable to large-scale manufacturing, and clinical studies investigating the drug safety and pharmacokinetics are ongoing.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model.
- Record: found
- Abstract: found
- Article: not found
Ebola RNA Persistence in Semen of Ebola Virus Disease Survivors - Preliminary Report.
- Record: found
- Abstract: found
- Article: not found
