- Record: found
- Abstract: found
- Article: found
Evaluation Instruments for Assessing Back Pain in Athletes: A Systematic Review Protocol
Read this article at
Abstract
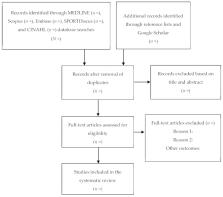
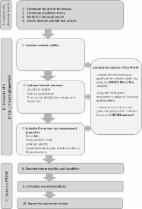
Back pain is a public health problem that affects adolescents and adults worldwide. However, studies on back pain present inconsistent findings in part due to the use of different instruments, especially for athletes. Therefore, the objective of this systematic review protocol was to map the existing evidence on such tools. The systematic review will be conducted according to PRISMA guidelines. Five electronic databases, Embase, MEDLINE, SPORTDiscus, CINAHL, and Scopus will be searched. This review includes studies that investigated prevalence, incidence, and other variables. Titles and abstracts will be selected. Two independent reviewers will read the articles carefully and discrepancies, if any, will be dealt with by a third reviewer. All steps will be completed with Rayyan for systematic reviews and the methodological quality will be analyzed with a COSMIN checklist. Discussion: This systematic review will gather evidence on tools that assess back pain in athletes. The findings may indicate the most appropriate tools for assessing back pain. They will contribute to better reliability, safe measurements, and help to standardize a comparison tool between different studies. They will also assist in the development of specific tools for athletes. Registration: This review was submitted and registered under CRD42020201299 in PROSPERO.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: found
Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement
- Record: found
- Abstract: not found
- Article: not found
EuroQol - a new facility for the measurement of health-related quality of life
- Record: found
- Abstract: found
- Article: found