- Record: found
- Abstract: found
- Article: not found
The effect of COVID-19 pandemic restrictions on neovascular AMD patients treated with treat-and-extend protocol
Read this article at
Abstract
Purpose
To investigate the adherence rate of neovascular age-related macular degeneration (nAMD) patients in treat-and-extend (TAE) protocol to their anti-vascular endothelial growth factor (anti-VEGF) intravitreal injection (IVI) appointments and to evaluate the functional and anatomical outcomes of the patients who attended and did not attend their IVI appointments during the coronavirus disease 2019 (COVID-19) restriction period (RP).
Methods
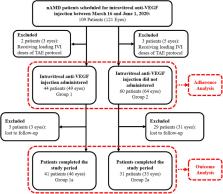
The patients with nAMD having IVI appointments between March 16 and June 1, 2020 (RP in Turkey) were included in this retrospective study. For adherence analysis, the patients who attended (Group 1, n = 44) and who did not attend (Group 2, n = 60) their IVI appointment visits during the RP ( V RP) were evaluated according to their last visit before the RP ( V 0). For outcome analysis, the patients who attend V RP and have follow-up (Group 1a, 46 eyes) and who did not attend V RP but later attended for follow-up (Group 2a, 33 eyes) were evaluated for functional (best-corrected visual acuity, BCVA [logMAR]) and anatomical (optical coherence tomography [OCT] disease activity) outcomes at the first visit after RP ( V 1) and last visit within six months after RP ( V 2). Patients received a complete ophthalmologic evaluation with anti-VEGF (Aflibercept) IVI administration at all visits.
Results
The adherence rate of the patients to V RP was 42.3% (44/104). The patients in Group 1 were significantly younger (mean ± SD years, 71.0 ± 8.1 vs. 74.7 ± 8.0, p = 0.024), had better median [IQR] BCVA at their first presentation (0.30 [0.54] vs. 0.61 [1.08], p = 0.023) and V 0 (0.40 [0.48] vs. 0.52 [0.70], p = 0.031), and had less hypertension (36.4% vs. 58.3%, p = 0.044) than Group 2. The mean ± SD delay of planned IVI at V RP in Group 2a was 13.9 ± 6.2 weeks. Disease activity in OCT was significantly higher in Group 2a than Group 1a at V 1 (60.6% vs. 32.6%, p = 0.025). In Group 2a, the median (IQR) BCVA was significantly worse at V 1 (0.70 [0.58]) and V 2 (0.70 [0.59]) than V 0 (0.52 [0.40], p = 0.047 and p = 0.035, respectively).
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China
- Record: found
- Abstract: found
- Article: not found
Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?
- Record: found
- Abstract: found
- Article: not found
