- Record: found
- Abstract: not found
- Article: not found
Discovering drugs to treat coronavirus disease 2019 (COVID-19)
Publication date Created:
2020
Publication date (Print):
February 29 2020
Journal:
Drug Discoveries & Therapeutics
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
The SARS-CoV-2 virus emerged in December 2019 and then spread rapidly worldwide, particularly
to China, Japan, and South Korea. Scientists are endeavoring to find antivirals specific
to the virus. Several drugs such as chloroquine, arbidol, remdesivir, and favipiravir
are currently undergoing clinical studies to test their efficacy and safety in the
treatment of coronavirus disease 2019 (COVID-19) in China; some promising results
have been achieved thus far. This article summarizes agents with potential efficacy
against SARS-CoV-2.
Related collections
Most cited references6
- Record: found
- Abstract: found
- Article: found
Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro
Manli Wang, Ruiyuan Cao, Leike Zhang … (2020)
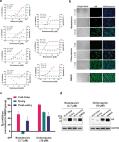
Dear Editor, In December 2019, a novel pneumonia caused by a previously unknown pathogen emerged in Wuhan, a city of 11 million people in central China. The initial cases were linked to exposures in a seafood market in Wuhan. 1 As of January 27, 2020, the Chinese authorities reported 2835 confirmed cases in mainland China, including 81 deaths. Additionally, 19 confirmed cases were identified in Hong Kong, Macao and Taiwan, and 39 imported cases were identified in Thailand, Japan, South Korea, United States, Vietnam, Singapore, Nepal, France, Australia and Canada. The pathogen was soon identified as a novel coronavirus (2019-nCoV), which is closely related to sever acute respiratory syndrome CoV (SARS-CoV). 2 Currently, there is no specific treatment against the new virus. Therefore, identifying effective antiviral agents to combat the disease is urgently needed. An efficient approach to drug discovery is to test whether the existing antiviral drugs are effective in treating related viral infections. The 2019-nCoV belongs to Betacoronavirus which also contains SARS-CoV and Middle East respiratory syndrome CoV (MERS-CoV). Several drugs, such as ribavirin, interferon, lopinavir-ritonavir, corticosteroids, have been used in patients with SARS or MERS, although the efficacy of some drugs remains controversial. 3 In this study, we evaluated the antiviral efficiency of five FAD-approved drugs including ribavirin, penciclovir, nitazoxanide, nafamostat, chloroquine and two well-known broad-spectrum antiviral drugs remdesivir (GS-5734) and favipiravir (T-705) against a clinical isolate of 2019-nCoV in vitro. Standard assays were carried out to measure the effects of these compounds on the cytotoxicity, virus yield and infection rates of 2019-nCoVs. Firstly, the cytotoxicity of the candidate compounds in Vero E6 cells (ATCC-1586) was determined by the CCK8 assay. Then, Vero E6 cells were infected with nCoV-2019BetaCoV/Wuhan/WIV04/2019 2 at a multiplicity of infection (MOI) of 0.05 in the presence of varying concentrations of the test drugs. DMSO was used in the controls. Efficacies were evaluated by quantification of viral copy numbers in the cell supernatant via quantitative real-time RT-PCR (qRT-PCR) and confirmed with visualization of virus nucleoprotein (NP) expression through immunofluorescence microscopy at 48 h post infection (p.i.) (cytopathic effect was not obvious at this time point of infection). Among the seven tested drugs, high concentrations of three nucleoside analogs including ribavirin (half-maximal effective concentration (EC50) = 109.50 μM, half-cytotoxic concentration (CC50) > 400 μM, selectivity index (SI) > 3.65), penciclovir (EC50 = 95.96 μM, CC50 > 400 μM, SI > 4.17) and favipiravir (EC50 = 61.88 μM, CC50 > 400 μM, SI > 6.46) were required to reduce the viral infection (Fig. 1a and Supplementary information, Fig. S1). However, favipiravir has been shown to be 100% effective in protecting mice against Ebola virus challenge, although its EC50 value in Vero E6 cells was as high as 67 μM, 4 suggesting further in vivo studies are recommended to evaluate this antiviral nucleoside. Nafamostat, a potent inhibitor of MERS-CoV, which prevents membrane fusion, was inhibitive against the 2019-nCoV infection (EC50 = 22.50 μM, CC50 > 100 μM, SI > 4.44). Nitazoxanide, a commercial antiprotozoal agent with an antiviral potential against a broad range of viruses including human and animal coronaviruses, inhibited the 2019-nCoV at a low-micromolar concentration (EC50 = 2.12 μM; CC50 > 35.53 μM; SI > 16.76). Further in vivo evaluation of this drug against 2019-nCoV infection is recommended. Notably, two compounds remdesivir (EC50 = 0.77 μM; CC50 > 100 μM; SI > 129.87) and chloroquine (EC50 = 1.13 μM; CC50 > 100 μM, SI > 88.50) potently blocked virus infection at low-micromolar concentration and showed high SI (Fig. 1a, b). Fig. 1 The antiviral activities of the test drugs against 2019-nCoV in vitro. a Vero E6 cells were infected with 2019-nCoV at an MOI of 0.05 in the treatment of different doses of the indicated antivirals for 48 h. The viral yield in the cell supernatant was then quantified by qRT-PCR. Cytotoxicity of these drugs to Vero E6 cells was measured by CCK-8 assays. The left and right Y-axis of the graphs represent mean % inhibition of virus yield and cytotoxicity of the drugs, respectively. The experiments were done in triplicates. b Immunofluorescence microscopy of virus infection upon treatment of remdesivir and chloroquine. Virus infection and drug treatment were performed as mentioned above. At 48 h p.i., the infected cells were fixed, and then probed with rabbit sera against the NP of a bat SARS-related CoV 2 as the primary antibody and Alexa 488-labeled goat anti-rabbit IgG (1:500; Abcam) as the secondary antibody, respectively. The nuclei were stained with Hoechst dye. Bars, 100 μm. c and d Time-of-addition experiment of remdesivir and chloroquine. For “Full-time” treatment, Vero E6 cells were pre-treated with the drugs for 1 h, and virus was then added to allow attachment for 2 h. Afterwards, the virus–drug mixture was removed, and the cells were cultured with drug-containing medium until the end of the experiment. For “Entry” treatment, the drugs were added to the cells for 1 h before viral attachment, and at 2 h p.i., the virus–drug mixture was replaced with fresh culture medium and maintained till the end of the experiment. For “Post-entry” experiment, drugs were added at 2 h p.i., and maintained until the end of the experiment. For all the experimental groups, cells were infected with 2019-nCoV at an MOI of 0.05, and virus yield in the infected cell supernatants was quantified by qRT-PCR c and NP expression in infected cells was analyzed by Western blot d at 14 h p.i. Remdesivir has been recently recognized as a promising antiviral drug against a wide array of RNA viruses (including SARS/MERS-CoV 5 ) infection in cultured cells, mice and nonhuman primate (NHP) models. It is currently under clinical development for the treatment of Ebola virus infection. 6 Remdesivir is an adenosine analogue, which incorporates into nascent viral RNA chains and results in pre-mature termination. 7 Our time-of-addition assay showed remdesivir functioned at a stage post virus entry (Fig. 1c, d), which is in agreement with its putative anti-viral mechanism as a nucleotide analogue. Warren et al. showed that in NHP model, intravenous administration of 10 mg/kg dose of remdesivir resulted in concomitant persistent levels of its active form in the blood (10 μM) and conferred 100% protection against Ebola virus infection. 7 Our data showed that EC90 value of remdesivir against 2019-nCoV in Vero E6 cells was 1.76 μM, suggesting its working concentration is likely to be achieved in NHP. Our preliminary data (Supplementary information, Fig. S2) showed that remdesivir also inhibited virus infection efficiently in a human cell line (human liver cancer Huh-7 cells), which is sensitive to 2019-nCoV. 2 Chloroquine, a widely-used anti-malarial and autoimmune disease drug, has recently been reported as a potential broad-spectrum antiviral drug. 8,9 Chloroquine is known to block virus infection by increasing endosomal pH required for virus/cell fusion, as well as interfering with the glycosylation of cellular receptors of SARS-CoV. 10 Our time-of-addition assay demonstrated that chloroquine functioned at both entry, and at post-entry stages of the 2019-nCoV infection in Vero E6 cells (Fig. 1c, d). Besides its antiviral activity, chloroquine has an immune-modulating activity, which may synergistically enhance its antiviral effect in vivo. Chloroquine is widely distributed in the whole body, including lung, after oral administration. The EC90 value of chloroquine against the 2019-nCoV in Vero E6 cells was 6.90 μM, which can be clinically achievable as demonstrated in the plasma of rheumatoid arthritis patients who received 500 mg administration. 11 Chloroquine is a cheap and a safe drug that has been used for more than 70 years and, therefore, it is potentially clinically applicable against the 2019-nCoV. Our findings reveal that remdesivir and chloroquine are highly effective in the control of 2019-nCoV infection in vitro. Since these compounds have been used in human patients with a safety track record and shown to be effective against various ailments, we suggest that they should be assessed in human patients suffering from the novel coronavirus disease. Supplementary information Supplementary information, Materials and Figures
- Record: found
- Abstract: found
- Article: not found
First Case of 2019 Novel Coronavirus in the United States
Michelle Holshue, Chas DeBolt, Scott Lindquist … (2020)
Summary An outbreak of novel coronavirus (2019-nCoV) that began in Wuhan, China, has spread rapidly, with cases now confirmed in multiple countries. We report the first case of 2019-nCoV infection confirmed in the United States and describe the identification, diagnosis, clinical course, and management of the case, including the patient’s initial mild symptoms at presentation with progression to pneumonia on day 9 of illness. This case highlights the importance of close coordination between clinicians and public health authorities at the local, state, and federal levels, as well as the need for rapid dissemination of clinical information related to the care of patients with this emerging infection.
- Record: found
- Abstract: found
- Article: not found
SARS: Systematic Review of Treatment Effects
Lauren J. Stockman, Richard Bellamy, Paul Garner (2006)
Introduction The severe acute respiratory syndrome (SARS) is a febrile respiratory illness primarily transmitted by respiratory droplets or close personal contact. A global outbreak of SARS between March 2003 and July 2003 caused over 8,000 probable or confirmed cases and 774 deaths [1]. The causative organism has been identified as a novel coronavirus (SARS-CoV) [2–4]. The overall mortality during the outbreak was estimated at 9.6% [5,6]. The overriding clinical feature of SARS is the rapidity with which many patients develop symptoms of acute respiratory distress syndrome (ARDS). This complication occurred in approximately 16% of all patients with SARS, and when it occurred was associated with a mortality rate of 50% [7,8]. At the time of the SARS epidemic it was not known what treatments would reduce SARS-related illness and deaths. Because the urgency of the international outbreak did not allow time for efficacy studies, physicians in Canada and Hong Kong treated the earliest patients with intravenous ribavirin, based on its broad-spectrum antiviral activity [9,10]. Corticosteroids and immune-modulating agents were often prescribed empirically. Soon after SARS-CoV was identified as the causative agent, antiviral screening programs were initiated; these programs reported several antiviral agents that inhibited SARS-CoV replication in vitro. These results led to the experimental use of protease inhibitors and interferon alpha (IFN-α) in the treatment of patients. The most commonly used treatments for SARS are associated with adverse effects when used for other conditions (Table S1). In October 2003, the WHO established an International SARS Treatment Study Group, consisting of experts experienced in managing SARS. The group recommended a systematic review of potential treatment options to identify the targets for proper evaluation in trials should the disease recur [11]. This paper reports on this systematic review designed to summarise available evidence on the effects of ribavirin, lopinavir and ritonavir (LPV/r), corticosteroids, type I IFN, intravenous immunoglobulin (IVIG), or convalescent plasma in relation to (1) SARS-CoV replication inhibition in vitro; (2) mortality or morbidity in SARS patients; and (3) effects on ARDS in adult patients. Methods We prepared a protocol that defined our scope, inclusion criteria, and outcomes to be assessed. The interventions we included were defined by the WHO: ribavirin, LPV/r, corticosteroids, type I IFN, convalescent plasma, or IVIG. The types of study we included were: (1) in vitro studies, in which the authors examined inhibition of SARS-CoV viral replication, and data from an assay in human or animal cell line; (2) in vivo studies, which included randomised controlled trial (RCT), or prospective uncontrolled study design, or retrospective cohort design, or case-control design, or a case series, and patients treated for SARS, and ten or more patients; and (3) studies of ARDS that included RCT, or systematic review, and treatment for ARDS or acute lung injury, and 20 or more patients. In February 2005, we systematically searched the literature databases MEDLINE, EMBASE, BIOSIS, and the Cochrane Central Register of Controlled Trials (CENTRAL) for articles that included the selected treatments (Table S2). The full text of each identified study was retrieved and each was independently reviewed by two authors (LS and RB). Publications in Chinese were selected after review of the English abstract. Unpublished data were not sought, as the task of summarising existing published data was extensive and the International SARS Treatment Group indicated that much of the clinical data had already been published. We used the QUOROM checklist to help ensure the quality of this review (Table S3). Data from the full text of studies in English were extracted independently by two authors (LS and RB). Data from the Chinese literature were extracted with the assistance of a translator. Because the Chinese articles were reviewed by only one author, the consistency of the translated information with that from English articles was maintained by subsequent discussion with the translator to verify the extracted data. We established explicit criteria to assess the level of evidence for each human treatment study (Box 1). Since the treatments chosen for evaluation were often given in combination, evidence was classified by the treatment that was given to all patients in the cohort or given to some with the author's intention of studying its effects. If putative effects within a study included several drugs, then we extracted data for each intervention. The level of evidence was independently classified by two authors (LS and RB). Chinese studies were appraised and classified in the same way using translated information extracted from each report. Discrepancies were resolved by consensus. Box 1. Categories of Evidence Defined for In Vivo Studies of Treatments in SARS Patients “Inconclusive” if a study could not be used to inform a decision about treatment efficacy due to having either outcomes which were not reported consistently, an inconsistent treatment regimen, no control group or a control group which was a likely source of bias. A control group was considered a likely source of bias if there were differences in co-morbidities, sex, age and markers of severe disease compared to the treatment group. “Possible harm” if a study reported adverse effects of treatment that were consistent with adverse effects reported with the use of the drug in the treatment of other conditions. Evidence of direct causality was not required. A study could be classified as suggesting possible harm from the drug even if the study had methodological weaknesses. “Possible benefit” if a study had evidence of benefit for an important outcome measure which was recorded consistently (e.g., case fatality, need for mechanical ventilation, duration of hospitalization, frequency of ARDS) in patients treated in a defined way compared to a valid control group. A control group was considered valid if randomized, or if patient characteristics and illness severity were comparable to the treatment group. Evidence of direct causality was not required. “Definite harm” if a study contained statistically significant evidence of harm demonstrated in a double-blind randomized trial, which did not contain serious methodological weaknesses. “Definite benefit” if a study contained statistically significant evidence of harm demonstrated in a double-blind randomized trial, which did not contain serious methodological weaknesses. Results In vitro evidence was available in 15 studies. Clinical evidence of SARS treatment in humans was reported in 54 studies (37 in English, 17 in Chinese). Three studies addressed treatment of ARDS (Figure 1). Ribavirin In vitro. We found six studies that described the antiviral effect of ribavirin in vitro (Table S4); four showed an antiviral effect (Table S5). A synergistic antiviral effect between ribavirin and type I IFN (IFN-β1a or leukocytic IFN-α) was described in two studies performed in human cell lines and Vero cell lines [12,13]. In SARS patients. We found 24 studies that described ribavirin treatment in cohorts larger than ten patients (Table S6). Our formal assessment classified 20 studies as “inconclusive,” due to study design or because the effect of ribavirin could not be distinguished from the effects of other treatments (such as steroids and antiviral drugs). Four publications presented evidence of possible harm (14–17). Three of these studies, each of which included over 100 patients, documented a fall in haemoglobin levels after ribavirin treatment when compared to levels in patients before treatment [14–16]. Of patients treated with ribavirin, 49/138 to 67/110 (36%–61%) developed haemolytic anaemia, a recognised complication with this drug, although it is not possible to rule out the possibility that SARS-CoV infection caused the haemolytic anaemia, as there is no control group. One study noted that over 29% of SARS patients had some degree of liver dysfunction indicated by ALT levels higher than normal, and the number of patients with this complication increased to over 75% after ribavirin treatment (Table S7) [17]. In the Chinese literature six additional reports described patients with SARS treated with ribavirin (often with steroids). These six reports were determined to be inconclusive in the evaluation of treatment for SARS (Tables S8 and S9). LPV/r In vitro. Of three studies, two demonstrated that lopinavir inhibits cytopathic effects of SARS-CoV in fetal rhesus monkey kidney cells (Table S4). One study showed detectable but reduced activity in Vero-E6 cells [13], and one study concluded that neither lopinavir nor ritonavir had an effect [18]. A synergistic effect of lopinavir with ribavirin has been reported (Table S5). In SARS patients. We found two studies of LPV/r (lopinavir 400 mg with ritonavir 100 mg orally every 12 h) in cohorts larger than ten patients (Table S6). Patients also received ribavirin and corticosteroids. LPV/r use was compared among three groups of patients: those who received it as an early SARS treatment, those who received it as a late treatment, and those who did not receive it at all. When LPV/r was added as an initial treatment to ribavirin and corticosteroid therapy, the death rate was lower than among those who received ribavirin and corticosteroids (1/44 [2.3%] versus 99/634 [15.6%]; p < 0.05) [19]. A second study of this regimen reported fewer episodes of ARDS or death compared with historical controls who had not received LPV/r (1/41 [2.4%] versus 32/111 [28.8%]; p < 0.001) (Table S7) [20]. Both studies were determined to be inconclusive due to possible bias in the selection of control group or treatment allocation. No additional studies were identified from the Chinese literature. Corticosteroids In vitro. No studies were found on the cytopathic effect of corticosteroids alone against SARS-CoV. Corticosteroids act as immunomodulatory agents, and therefore studies to measure direct antiviral effects in vitro were not expected. In SARS patients. Fifteen articles examined corticosteroid treatment in ten or more patients. Of these cohorts 13 were also treated with ribavirin (Table S6). We determined that 13 of the 15 studies were inconclusive. Of these, in an uncontrolled and nonrandomised study, 95/107 (89%) of patients treated with high-dose methylprednisolone (0.5–1 mg/kg prednisolone on day 3 of illness, followed by hydrocortisolone 100 mg every 8 h, and pulse-doses of methylprednisolone 0.5 g IV for 3 d) after the first week of illness recovered from progressive lung disease (Table S7) [16]. Two studies contained evidence of possible harm from corticosteroids [21,22]. One measured SARS-CoV plasma viral load across time after fever onset in a randomized, double-blind, placebo-controlled trial; corticosteroid use within the first week of illness was associated with delayed viral clearance. The other study, which was case-controlled, found that patients with psychosis received higher cumulative doses of steroids than patients without psychosis (10,975 mg versus 6,780 mg; p = 0.017) [22]. In the Chinese literature, we found 14 reports in which steroids were used (Table S8 and Table S9). Twelve studies were inconclusive and two showed possible harm. One study reported diabetes onset associated with methylprednisolone treatment [23]. Another study (an uncontrolled, retrospective study of 40 SARS patients) reported avascular necrosis and osteoporosis among corticosteroid-treated SARS patients [24]. In ARDS patients. Three clinical trials examined the effect of corticosteroids on mortality in patients with established ARDS (Table S10). In two trials, high-dose methylprednisolone given for approximately 2 d was not effective for early ARDS [25,26]. One small RCT that used a regimen of lower dose methylprednisolone (2 mg/kg per day), tapered after 2 wk, showed possible evidence of ARDS improvement (Table S11) [27]. IFN Type I In vitro. Twelve in vitro studies with data on the antiviral effect of IFN type I have been reported, and all demonstrated an antiviral effect against SARS-CoV (six for IFN-α and ten for IFN-β) (Tables S4 and S5). Antiviral effects have been demonstrated in monkey (Vero; Vero-E6), fetal rhesus monkey kidney (fRhK-4), and human (Caco2, CL14, and HPEK) cell lines. Three reports presented evidence that IFN-β was superior against SARS-CoV compared to IFN-α and found rIFN-α2 virtually ineffective against SARS-CoV compared to other IFNs [28]. Synergistic effects were reported for leukocytic IFN-α with ribavirin [13], IFN-β with ribavirin [12,13] and IFN-β with IFN-γ [28,29]. In SARS patients. Two studies of IFN-α given with steroids and/or ribavirin were reported (Table S6). No significant difference was seen in outcome between IFN-α treatment group and those treated with other regimens. Results of both studies were inconclusive due to a lack of a consistent treatment regimen or suitable control group (Table S7). In the Chinese literature, one additional study reported the use of IFN-α as part of a regimen that included ribavirin and steroids [30]. We determined this study to be inconclusive because a variety of treatments given masked the effect of IFN-α alone (Table S8 and Table S9). Convalescent Plasma or Immunoglobulin In vitro. No studies were found on the cytopathic effect of this treatment on SARS-CoV. Convalescent plasma and IVIG act as immunomodulatory agents and therefore studies to measure direct antiviral effects in vitro were not expected. In SARS patients. Five studies of either IVIG or convalescent plasma treatment given in addition to steroids and ribavirin were reported for treatment of SARS (Table S6). These studies were inconclusive, because the effect of convalescent plasma or IVIG could not be discerned from effects of patient comorbidities, stage of illness, or effect of other treatments (Table S7). In the Chinese literature, two additional studies reported evidence on the effect of convalescent plasma as a treatment for SARS [30,31]. These studies were inconclusive (Table S8 and Table S9). Evidence collected on the benefit or harm of drugs used to treat SARS is summarized in Table 1. Discussion The rapid spread and subsequent control of SARS precluded controlled clinical treatment trials during the outbreak of 2002–2003. In this report we summarize the results of a systematic evaluation of the findings from published reports of treatments used for SARS during the epidemic. Publications from the Chinese literature were included to capture as much evidence as possible. We developed specific criteria (Box 1) to look for large, obvious effects of benefit, adverse or poor outcomes, or evidence of potential benefit that could be used to prioritise future research of SARS treatments. A summary of this evidence in SARS patients is shown in Table 1. Despite thirty reports of SARS-infected patients treated with ribavirin, there is no convincing evidence that it led to recovery. Haemolytic anaemia, a recognized side effect of this treatment, was observed in three studies. We would infer from these findings that any future use of ribavirin for SARS should be within the context of a controlled trial with close attention given to adverse effects. Corticosteroids were commonly prescribed to SARS patients with worsening pulmonary disease or progressing abnormalities on chest X-rays. Treatment regimens varied widely but can be classified into two groups, early treatment and rescue treatment given at a later stage of illness. It is difficult to make a clear recommendation about whether corticosteroids should be used to treat SARS-associated lung injury in any stage of illness, particularly as the drug is immunosuppressive and may delay viral clearance if given before viral replication is controlled [21]. Of added concern are infectious complications, avascular necrosis, and steroid-induced psychosis—recognized adverse effects of corticosteroid use. Fungal superinfection and aspergillosis have been noted in case reports and autopsy findings of SARS patients given corticosteroids at high doses or for prolonged periods [32,33]. This review has found evidence of avascular necrosis and steroid-induced psychosis in SARS patients. Seven studies of treatment with convalescent plasma or IVIG, three with IFN type I, and two with LPV/r were inconclusive by the criteria used in our analyses. Authors of four of the IVIG studies commented that patients seemed to improve upon treatment, but that more controlled trials of this approach are needed to provide evidence of an effect for SARS. Important caveats should be considered in this review. Most of the studies of SARS patients were descriptions of the natural course of the disease and had not been designed to reliably assess the effects of the treatments used. Patient characteristics such as age and presence of diabetes mellitus have been associated with severe disease and can confound treatment effects. A diagnostic test for early SARS illness was not validated or widely available, and in general, treatment was initiated once patients fulfilled a clinical and epidemiological case definition. It is possible that the inclusion of patients without laboratory confirmation of SARS-CoV infection in this review could cause an underestimate of any true effect of antiviral treatment on SARS. The variation in treatment regimens—particularly the wide range in doses, duration of therapy, and route of administration of ribavirin and corticosteroids—is a major obstacle to a clear interpretation of the data in this review. The nonstandardised collection of clinical information limits the conclusions that can be drawn from a retrospective analysis. We suggest that, in the event of a future outbreak of SARS-CoV or another novel agent, attempts be made to develop treatment protocols and to collect and contribute information for a standardized minimum dataset that could facilitate analysis of treatment outcomes among different settings. As observational studies pose problems of interpretation, the need is great for good-quality randomised trials, despite the difficulties in organising such trials. Supporting Information Table S1 Rationale for Treatments and Recognized Adverse Effects (55 KB DOC) Click here for additional data file. Table S2 Method of Systematic Review (A) Search strategy, step 1: Select the treatments. (B) Search strategy, step 2: Narrow the scope. (C) Inclusion criteria and information sought from each study. (45 KB DOC) Click here for additional data file. Table S3 QUOROM Statement (50 KB DOC) Click here for additional data file. Table S4 Description of SARS-CoV Replication Studies: Assay Type and Outcomes Measured (79 KB DOC) Click here for additional data file. Table S5 Results from SARS-CoV Replication Studies: Inhibition of SARS-CoV Replication (84 KB DOC) Click here for additional data file. Table S6 Description of Studies within SARS Patients (186 KB DOC) Click here for additional data file. Table S7 Results of Treatment within SARS Patients (English literature) (183 KB DOC) Click here for additional data file. Table S8 Description of Studies of SARS Patients (Chinese Literature) (108 KB DOC) Click here for additional data file. Table S9 Results of Treatment within SARS Patients (Chinese Literature) (93 KB DOC) Click here for additional data file. Table S10 Description of Studies of ARDS or ALI (50 KB DOC) Click here for additional data file. Table S11 Results of Treatment of ARDS or ALI (48 KB DOC) Click here for additional data file.
Author and article information
Journal
Title:
Drug Discoveries & Therapeutics
Abbreviated Title:
DD&T
Publisher:
International Research and Cooperation Association for Bio & Socio-Sciences Advancement
(IRCA-BSSA)
ISSN
(Print):
1881-7831
ISSN
(Electronic):
1881-784X
Publication date Created: 2020
Publication date
(Print):
February
29 2020
Volume: 14
Issue: 1
Pages: 58-60
Affiliations
Article
DOI: 10.5582/ddt.2020.01012
PubMed ID: 32147628
SO-VID: 6e79e793-03d3-4962-b202-dfdb22b2f558
Copyright © ©
2020
History
Data availability:
