- Record: found
- Abstract: found
- Article: found
COVID-19 mRNA vaccine in pregnancy: Results of the Swiss COVI-PREG registry, an observational prospective cohort study
Read this article at
Summary
Background
Pregnant individuals with coronavirus disease 2019 (COVID-19) are at increased risk of severe disease, prematurity, and stillbirth. In March 2021, vaccination for at risk pregnant women was recommended in Switzerland, expanding this to all pregnant women in May 2021. Our aim was to assess the safety of mRNA COVID-19 vaccines in pregnancy.
Methods
This multicentre prospective cohort study describes early adverse events and perinatal outcomes in pregnant women who received at least one dose of mRNA vaccine between March 1st and December 27th, 2021 in Switzerland, using the COVI-PREG registry. Early adverse events were collected at least one month following vaccine administration. Pregnancy and neonatal outcomes were extracted from medical records using the maternity discharge letters providing follow-up information up to 5 days after birth.
Findings
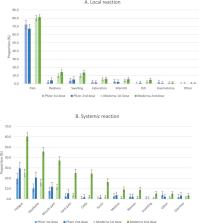
Of 1012 vaccinated women, 894 (88·3%) received both injections during pregnancy, with BNT162b2 ( n = 271) or mRNA-1273 ( n = 623) vaccines. Local events (mainly local pain) were reported in 81·3% and 80·5% after the first and second doses. Rates of systemic reactions (mainly fatigue and headache) were similar after the first dose and most frequent after the second dose of mRNA-1273. Of the 1012 women, four (0·4%; 95%CI [0·1-1·0]) severe early adverse events occurred: pulmonary embolism, preterm premature rupture of membranes, isolated fever with hospitalisation, and herpes zoster. Of 107 patients vaccinated before 14 weeks, one (0·9%; 95%CI [0·0-5·1]) early spontaneous abortions was reported (8 weeks). Of 228 vaccinated before 20 weeks one (0·4%; 95%CI [0·0-2·4]) late spontaneous abortion was reported (16 weeks). Of 513 women exposed before 37 weeks, 33 (6·4%; 95%CI [4·5-8·9]) delivered preterm. Among 530 patients exposed in pregnancy, no stillbirth was reported and 25 (4·7%; 95%CI [3·0-6·8]) neonates were admitted to intensive care unit.
Interpretation
Frequent local and systemic effects were described after exposure to mRNA COVID-19 vaccines during pregnancy but severe events were rare. Women vaccinated during pregnancy did not experience higher adverse pregnancy or neonatal outcomes when compared to historical data on background risks in the obstetric population.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
- Record: found
- Abstract: found
- Article: not found
Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine
- Record: found
- Abstract: found
- Article: not found