- Record: found
- Abstract: found
- Article: found
Bone morphogenetic protein 6–mediated crosstalk between endothelial cells and hepatocytes recapitulates the iron-sensing pathway in vitro
Read this article at
Abstract
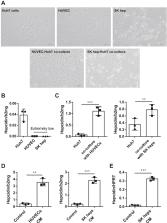
Liver sinusoidal endothelial cell–derived bone morphogenetic protein 6 (BMP6) and the BMP6–small mothers against decapentaplegic homolog (SMAD) signaling pathway are essential for the expression of hepcidin, the secretion of which is considered the systemic master switch of iron homeostasis. However, there are continued controversies related to the strong and direct suppressive effect of iron on hepatocellular hepcidin in vitro in contrast to in vivo conditions. Here, we directly studied the crosstalk between endothelial cells (ECs) and hepatocytes using in vitro coculture models that mimic hepcidin signaling in vivo. Huh7 cells were directly cocultured with ECs, and EC conditioned media (CM) were also used to culture Huh7 cells and primary mouse hepatocytes. To explore the reactions of ECs to surrounding iron, they were grown in the presence of ferric ammonium citrate and heme, two iron-containing molecules. We found that both direct coculture with ECs and EC-CM significantly increased hepcidin expression in Huh7 cells. The upstream SMAD pathway, including phosphorylated SMAD1/5/8, SMAD1, and inhibitor of DNA binding 1, was induced by EC-CM, promoting hepcidin expression. Efficient blockage of this EC-mediated hepcidin upregulation by an inhibitor of the BMP6 receptor ALK receptor tyrosine kinase 2/3 or BMP6 siRNA identified BMP6 as a major hepcidin regulator in this coculture system, which highly fits the model of hepcidin regulation by iron in vivo. In addition, EC-derived BMP6 and hepcidin were highly sensitive to levels of not only ferric iron but also heme as low as 500 nM. We here establish a hepatocyte–endothelial coculture system to fully recapitulate iron regulation by hepcidin using EC-derived BMP6.
Related collections
Most cited references52
- Record: found
- Abstract: found
- Article: not found
Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization.
- Record: found
- Abstract: found
- Article: not found
Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein.
- Record: found
- Abstract: found
- Article: not found