- Record: found
- Abstract: found
- Article: found
Human Amnion Epithelial Cells (AECs) Respond to the FSL-1 Lipopeptide by Engaging the NLRP7 Inflammasome

Read this article at
Abstract
Context and Objectives: Inflammation is the leading mechanism involved in both physiological and pathological rupture of fetal membranes. Our aim was to obtain a better characterization of the inflammasome-dependent inflammation processes in these tissues, with a particular focus on the nucleotide-binding oligomerization domain (NOD)–like receptor, pyrin domain containing protein 7 (NLRP7) inflammasome.
Methods: The presence of NLRP7 inflammasome actors [NLRP7, apoptosis-associated speck–like protein containing a CARD domain (ASC), and caspase-1] was confirmed by reverse transcriptase–polymerase chain reaction (RT-PCR) in human amnion and choriodecidua at the three trimesters and at term. The protein concentrations were then determined by enzyme-linked immunosorbent assay in term tissues, with or without labor. The presence of Mycoplasma salivarium and Mycoplasma fermentans in human fetal membranes was investigated using a PCR approach. Human amnion epithelial cells (AECs) were treated for 4 or 20 h with fibroblast-stimulating lipopeptide-1 (FSL-1), a M. salivarium–derived ligand. Transcripts and proteins quantity was then measured by RT–quantitative PCR and Western blotting, respectively. NLRP7 and ASC colocalization was confirmed by immunofluorescence. Western blots allowed analysis of pro–caspase-1 and gasdermin D cleavage.
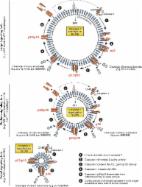
Results: NLRP7, ASC, and caspase-1 transcripts were expressed in both sheets of human fetal membranes during all pregnancy stages, but only ASC protein expression was increased with labor. In addition, M. salivarium and M. fermentans were detected for the first time in human fetal membranes. NLRP7 and caspase-1 transcripts, as well as NLRP7, ASC, and pro–caspase-1 protein levels, were increased in FSL-1–treated AECs. The NLRP7 inflammasome assembled around the nucleus, and pro–caspase-1 and gasdermin D were cleaved into their mature forms after FSL-1 stimulation.
Conclusion: Two new mycoplasmas, M. salivarium and M. fermentans, were identified in human fetal membranes, and a lipopeptide derived from M. salivarium was found to induce NLRP7 inflammasome formation in AECs.
Related collections
Most cited references84

- Record: found
- Abstract: found
- Article: found
GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death
- Record: found
- Abstract: found
- Article: not found
Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages.
- Record: found
- Abstract: found
- Article: found