- Record: found
- Abstract: not found
- Article: not found
An inhibitor of oxidative phosphorylation exploits cancer vulnerability
Jennifer R. Molina ,
Yuting Sun ,
Marina Protopopova ,
Sonal Gera ,
Madhavi Bandi ,
Christopher Bristow ,
Timothy McAfoos ,
Pietro Morlacchi ,
Jeffrey Ackroyd ,
Ahmed-Noor A. Agip ,
Gheath Al-Atrash ,
John Asara ,
Jennifer Bardenhagen ,
Caroline C. Carrillo ,
Christopher Carroll ,
Edward Chang ,
Stefan Ciurea ,
Jason B. Cross ,
Barbara Czako ,
Angela Deem ,
Naval Daver ,
John Frederick de Groot ,
Jian-Wen Dong ,
Ningping Feng ,
Guang Gao ,
Jason Gay ,
Mary Geck Do ,
Jennifer Greer ,
Virginia Giuliani ,
Jing Han ,
Lina Han ,
Verlene K. Henry ,
Judy Hirst ,
Sha Huang ,
Yongying Jiang ,
Zhijun Kang ,
Tin Khor ,
Sergej Konoplev ,
Yu-Hsi Lin ,
Gang Liu ,
Alessia Lodi ,
Timothy Lofton ,
Helen Ma ,
Mikhila Mahendra ,
Polina Matre ,
Robert Mullinax ,
Michael Peoples ,
Alessia Petrocchi ,
Jaime Rodriguez-Canale ,
Riccardo Serreli ,
Thomas Shi ,
Melinda Smith ,
Yoko Tabe ,
Jay Theroff ,
Stefano Tiziani ,
Quanyun Xu ,
Qi Zhang ,
Florian Muller ,
Ronald A. DePinho ,
Carlo Toniatti ,
Giulio F. Draetta ,
Timothy P. Heffernan ,
Marina Konopleva ,
Philip Jones ,
M. Emilia Di Francesco ,
Joseph R. Marszalek
June 11 2018
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Related collections
Most cited references37
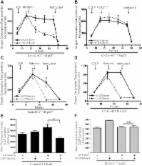
- Record: found
- Abstract: found
- Article: found
Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis
- Record: found
- Abstract: found
- Article: not found
Streaming fragment assignment for real-time analysis of sequencing experiments
Adam Roberts, Lior Pachter (2012)
- Record: found
- Abstract: found
- Article: not found
A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue.
Min lan Yuan, Susanne B Breitkopf, Xuemei Yang … (2012)
