- Record: found
- Abstract: found
- Article: not found
The role of HMGB1 in COVID‐19‐induced cytokine storm and its potential therapeutic targets: A review

Read this article at
Abstract
Hyperinflammation characterized by elevated proinflammatory cytokines known as ‘cytokine storms’ is the major cause of high severity and mortality seen in COVID‐19 patients. The pathology behind the cytokine storms is currently unknown. Increased HMGB1 levels in serum/plasma of COVID‐19 patients were reported by many studies, which positively correlated with the level of proinflammatory cytokines. Dead cells following SARS‐CoV‐2 infection might release a large amount of HMGB1 and RNA of SARS‐CoV‐2 into extracellular space. HMGB1 is a well‐known inflammatory mediator. Additionally, extracellular HMGB1 might interact with SARS‐CoV‐2 RNA because of its high capability to bind with a wide variety of molecules including nucleic acids and could trigger massive proinflammatory immune responses. This review aimed to critically explore the many possible pathways by which HMGB1‐SARS‐CoV‐2 RNA complexes mediate proinflammatory responses in COVID‐19. The contribution of these pathways to impair host immune responses against SARS‐CoV‐2 infection leading to a cytokine storm was also evaluated. Moreover, since blocking the HMGB1‐SARS‐CoV‐2 RNA interaction might have therapeutic value, some of the HMGB1 antagonists have been reviewed. The HMGB1‐ SARS‐CoV‐2 RNA complexes might trigger endocytosis via RAGE which is linked to lysosomal rupture, PRRs activation, and pyroptotic death. High levels of the proinflammatory cytokines produced might suppress many immune cells leading to uncontrolled viral infection and cell damage with more HMGB1 released. Altogether these mechanisms might initiate a proinflammatory cycle leading to a cytokine storm. HMGB1 antagonists could be considered to give benefit in alleviating cytokine storms and serve as a potential candidate for COVID‐19 therapy.
Abstract
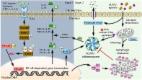
Extracellular High Mobility Group Box 1 (HMGB1), which could be a crucial factor behind the COVID‐19‐induced cytokine storm, is long‐known as a potent activator of TLR 4‐mediated proinflammatory cytokines release. HMGB1‐RNA SARS‐CoV‐2 complexes via RAGE might trigger inflammasome activation, pyroptotic cell death, and the initiation of coagulation, followed by a massive production of proinflammatory cytokines which suppressed many immune cells leading to uncontrolled viral infection and cells damage, causing more HMGB1 release. This vicious cycle potentially induced a cytokine storm.
Related collections
Most cited references136
- Record: found
- Abstract: found
- Article: not found
The trinity of COVID-19: immunity, inflammation and intervention
- Record: found
- Abstract: found
- Article: found
NF-κB signaling in inflammation
- Record: found
- Abstract: found
- Article: not found

