- Record: found
- Abstract: found
- Article: found
Role of Protein Glycosylation in Host-Pathogen Interaction
Read this article at
Abstract
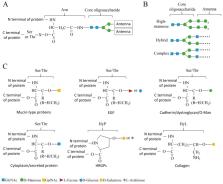
Host-pathogen interactions are fundamental to our understanding of infectious diseases. Protein glycosylation is one kind of common post-translational modification, forming glycoproteins and modulating numerous important biological processes. It also occurs in host-pathogen interaction, affecting host resistance or pathogen virulence often because glycans regulate protein conformation, activity, and stability, etc. This review summarizes various roles of different glycoproteins during the interaction, which include: host glycoproteins prevent pathogens as barriers; pathogen glycoproteins promote pathogens to attack host proteins as weapons; pathogens glycosylate proteins of the host to enhance virulence; and hosts sense pathogen glycoproteins to induce resistance. In addition, this review also intends to summarize the roles of lectin (a class of protein entangled with glycoprotein) in host-pathogen interactions, including bacterial adhesins, viral lectins or host lectins. Although these studies show the importance of protein glycosylation in host-pathogen interaction, much remains to be discovered about the interaction mechanism.
Related collections
Most cited references212
- Record: found
- Abstract: found
- Article: not found
Mitogen-activated protein kinases in innate immunity.
- Record: found
- Abstract: found
- Article: not found
Avian flu: influenza virus receptors in the human airway.
- Record: found
- Abstract: found
- Article: not found