Introduction
Percutaneous coronary intervention (PCI) is currently the primary treatment for acute coronary syndrome (ACS), one of the most serious threats to human health worldwide [1]. Very late stent thrombosis (VLST), defined as thrombosis occurring more than 1 year after stent implantation, is a rare but catastrophic complication of PCI that presents primarily as acute myocardial infarction (MI) or even sudden cardiac death [2]. The mechanism underlying VLST is complicated. VLST risk factors include patient- and lesion-related factors, procedural and stent-related factors, and pharmacotherapy-related factors [3]. Recent studies have suggested that: hypersensitivity and inflammation reactions, changes in shear stress, plaque rupture, and neointimal erosion may be involved in the development of VLST [3].
Emergent revascularization combined with optimized drug therapies have become the standard strategy for patients with VLST [4]. However, studies have provided limited information regarding the outcomes of patients with angiographically confirmed VLST as therapies have improved [5]. Therefore, we conducted a two-center registry study enrolling consecutive patients presenting with definite VLST, to identify the current incidence of major adverse cardiac events (MACE) after revascularization for VLST and the determinants of these events.
Methods
Patient Population
All consecutive patients with angiographically confirmed very late stent thrombosis (according to the Academic Research Consortium (ARC) stent thrombosis definitions [6]) at the First Hospital of Jilin University and First affiliated Hospital of Shantou University between January 1, 2014, and January 1, 2016, were enrolled (ClinicalTrials.gov: NCT03491891). This study was approved by the Institutional Review Board of the First Hospital of Jilin University (No. 2013256). All participants provided informed consent.
VLST was defined according to the ARC definition [6]. Coronary angiograms of both procedures (index PCI and PCI for VLST) were reviewed by two experienced interventional cardiologists independently. A consensus was established between reviewers or, in the event of divergent results, by a third reviewer.
Data Collection
Clinical follow-up information was obtained from hospital records or telephone interviews with the patients and their relatives every 3 months until 3 years after discharge. MACE were defined as nonfatal MI, recurrent of stent thrombosis, target vessel revascularization (TVR), heart failure requiring hospitalization, and all-cause death. Recurrent stent thrombosis was defined according to the ARC stent thrombosis definitions [6]. The diagnosis of myocardial infarction (MI) conformed to the 2012 ESC global definition of acute MI. TVR was defined as ischemia-driven PCI or coronary artery bypass grafting (CABG) performed in the same vessel as the index PCI.
Statistical Analysis
Data are summarized as numbers (and percentages) for categorical variables. Continuous variables are expressed as mean ± SD. Qualitative data were compared with chi-square or Fisher’s exact tests, whereas quantitative data were compared with the Mann-Whitney U test. All tests were two-sided and had a significance level of 0.05. Predictors of MACE were assessed with Cox regression, with univariate and multivariate analyses. Kaplan-Meier survival analyses were used to demonstrate the cumulative incidence of MACE and estimate MACE-free survival. Various correction methods were applied according to specific situations. Statistical analysis was performed in Statistical Package for Social Sciences version 23 (SPSS, Chicago, IL, USA).
Results
Patient Characteristics
In this follow-up study, 564 patients with an angiographically confirmed VLST were enrolled. The clinical characteristics of the patients are shown in Table 1. Angiographic and interventional parameters for both procedures are shown in Table 2. A total of 428 men (75.89%) were included in this study, and the average age was 61.61 ± 10.71 (29–91) years. The mean time from the index PCI to the occurrence of VLST was 1678.91 ± 1167.22 (365–5247) days. The clinical diagnosis for the index PCI was acute MI in 394 (69.86%) patients. Most patients (n = 546, 96.81%) had acute MI and underwent emergent PCI. Of these, 12 (2.12%) patients presented with cardiac arrest, and 18 (3.19%) patients had complications of cardiogenic shock. VLST occurred most frequently in the left anterior descending artery (LAD) (LAD, 51.06%; right coronary artery, 32.62%; circumflex, 16.31%). VLST was associated with a first-generation drug-eluting stent (DES) in 442 patients (78.37%) and a second-generation DES in 122 (21.63%) patients. Additionally, 222 (39.36%) patients were taking clopidogrel at the time of VLST, 302 (53.55%) patients were taking aspirin, and 218 (38.65%) patients were undergoing dual antiplatelet therapy (DAPT).
Patient Characteristics.
| Variables | Overall (n = 564) | Event free (n = 364) | With event (n = 200) | P value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 61.61 ± 10.71 | 60.18 ± 10.42 | 64.23 ± 10.00 | .001 |
| Male, n (%) | 428 (75.89%) | 282 (77.47%) | 146 (73%) | .401 |
| BMI (kg/m2) | 24.74 ± 2.73 | 24.96 ± 2.81 | 24.33 ± 2.54 | .070 |
| History of HT, n (%) | 308 (54.61%) | 192 (34.04%) | 116 (58%) | .072 |
| History of DM, n (%) | 210 (37.23%) | 122 (33.52%) | 88 (44%) | .397 |
| History of HL, n (%) | 74 (13.12%) | 44 (12.09%) | 30 (15%) | .081 |
| Current drinking, n (%) | 82 (14.54%) | 48 (13.19%) | 34 (17%) | .488 |
| Current smoking, n (%) | 336 (59.57%) | 206 (56.596%) | 130 (65%) | .379 |
| History of MI, n (%) | 384 (68.09%) | 250 (68.68%) | 134 (67%) | .379 |
| History of HF, n (%) | 62 (10.99%) | 14 (3.85%) | 48 (24%) | .772 |
| AMI as initial diagnosis, n (%) | 394 (69.86%) | 256 (70.33%) | 138 (69%) | .401 |
| STEMI as initial diagnosis, n (%) | 268 (47.52%) | 158 (43.41%) | 110 (55%) | .062 |
| Duration to occurrence of VLST (days) | 1678.91 ± 1165.14 | 1515.21 ± 1105.50 | 1976.84 ± 1222.16 | .001 |
| Cardiac arrest, n (%) | 12 (2.13%) | 6 (1.65%) | 6 (3%) | .484 |
| Cardiac shock, n (%) | 18 (3.19%) | 6 (1.65%) | 12 (6%) | .669 |
| Systolic pressure (mmHg) | 132.74 ± 20.85 | 133.46 ± 19.57 | 131.43 ± 23.04 | .623 |
| Diastolic pressure (mmHg) | 78.35 ± 11.36 | 78.86 ± 11.44 | 77.41 ± 11.22 | .572 |
| Heart rate (bpm) | 76.27 ± 14.22 | 75.13 ± 14.44 | 78.35 ± 13.63 | .019 |
| Killip ≥2, n (%) | 80 (14.18%) | 22 (6.04%) | 58 (29%) | <0.001 |
| Laboratory tests | ||||
| Peak troponin I (ng/mL) | 42.57 ± 83.98 | 33.04 ± 71.09 | 59.91 ± 101.54 | .023 |
| WBC (×109/L) | 9.54 ± 3.45 | 9.14 ± 3.02 | 10.28 ± 4.03 | .026 |
| RBC (×1012/L) | 4.62 ± 0.58 | 4.65 ± 0.59 | 4.57 ± 0.55 | .143 |
| HGB (g/L) | 140.48 ± 17.49 | 141.63 ± 17.58 | 138.39 ± 17.23 | .090 |
| Platelets (×109/L) | 213.73 ± 64.56 | 213.32 ± 64.86 | 214.47 ± 64.34 | .685 |
| TC (mmol/L) | 4.38 ± 1.33 | 4.38 ± 1.26 | 4.37 ± 1.45 | .716 |
| LDL (mmol/L) | 2.71 ± 1.05 | 2.71 ± 1.07 | 2.73 ± 1.01 | .787 |
| HDL (mmol/L) | 1.12 ± 0.26 | 1.12 ± 0.27 | 1.12 ± 0.25 | .843 |
| TG (mmol/L) | 2.04 ± 1.29 | 2.08 ± 1.38 | 1.96 ± 1.11 | .977 |
| Fasting blood glucose (mmol/L) | 7.17 ± 3.12 | 7.00 ± 3.18 | 7.49 ± 2.99 | .051 |
| HbA1c (%) | 6.54 ± 1.83 | 6.50 ± 1.92 | 6.61 ± 1.64 | .269 |
| Fibrinogen (g/L) | 3.37 ± 0.96 | 3.33 ± 0.93 | 3.45 ± 1.00 | .193 |
| eGFR (mL/min/1.73 m2) | 94.22 ± 21.43 | 97.34 ± 18.30 | 88.54 ± 25.32 | .001 |
| LVEF (%) | 51.21 ± 8.95 | 53.44 ± 7.25 | 47.14 ± 10.27 | <0.001 |
| Medications before VLST | ||||
| Aspirin, n (%) | 302 (53.55%) | 200 (54.95%) | 102 (51%) | .834 |
| Clopidogrel, n (%) | 222 (39.36%) | 154 (42.31%) | 68 (34%) | .525 |
| Statins, n (%) | 258 (45.74%) | 178 (48.9%) | 80 (40%) | .214 |
| Discharge medications (n = 548, 364, 184 per group) | ||||
| Aspirin, n (%) | 498 (90.88%) | 340 (93.41%) | 158 (85.87%) | .001 |
| Clopidogrel, n (%) | 268 (47.52%) | 182 (50%) | 86 (46.74%) | .041 |
| Ticagrelor, n (%) | 126 (22.34%) | 80 (21.98%) | 46 (25%) | .610 |
| DAPT, n (%) | 374 (66.31%) | 248 (68.13%) | 126 (68.48%) | .954 |
| Statins, n (%) | 460 (81.56%) | 306 (84.07%) | 154 (83.70%) | .937 |
| ACEI/ARB, n (%) | 292 (51.77%) | 202 (55.49%) | 90 (48.91%) | .366 |
| β blockers, n (%) | 336 (59.57%) | 242 (66.48%) | 94 (51.09%) | .302 |
ACEI, angiotensin converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor inhibitor; CABG, coronary artery bypass graft; DAPT, dual antiplatelet therapy; EES, everolimus eluting stent; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; HGB, hemoglobin; LAD, left anterior descending artery; LCX, left circumflex artery; LDL, low density lipoprotein; LM, left main; LVEF, left ventricular eject fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; RCA, right coronary artery; SES, sirolimus eluting stent; TCL, total cholesterol; TG, triglyceride; TIA, transient ischemic attack; TIMI, thrombolysis in myocardial infarction; WBC, white blood cell; VLST, very late stent thrombosis; ZES, zotarolimus eluting stent.
Angiographic and Interventional Characteristics of both Procedures.
| Variables | Overall (n = 564) | Event free (n = 364) | With event (n = 200) | P value |
|---|---|---|---|---|
| Parameters during index PCI | ||||
| No. of diseased vessels, n (%) | 2.20 ± 0.80 | 2.12 ± 0.81 | 2.34 ± 0.78 | .025 |
| PCI related vessel | ||||
| LAD, n (%) | 288 (51.06%) | 186 (51.10%) | 102 (51%) | .987 |
| LCX, n (%) | 92 (16.31%) | 62 (17.03%) | 30 (15%) | .658 |
| RCA, n (%) | 184 (32.62%) | 116 (31.87%) | 68 (34%) | .631 |
| Chronic total occlusion, n (%) | 32 (5.67%) | 22 (6.04%) | 10 (5%) | .005 |
| Severe calcification, n (%) | 14 (2.48%) | 8 (2.20%) | 6 (3%) | .989 |
| Severe tortuosity, n (%) | 12 (2.13%) | 6 (1.65%) | 6 (3%) | .666 |
| Ostial lesion, n (%) | 30 (5.32%) | 24 (6.59%) | 6 (3%) | .761 |
| Proximal lesion, n (%) | 406 (71.99%) | 260 (71.43%) | 146 (73%) | .198 |
| Bifurcation lesion, n (%) | 128 (22.70%) | 84 (23.08%) | 44 (22%) | .836 |
| Visual thrombosis, n (%) | 30 (5.32%) | 22 (6.04%) | 8 (4%) | .464 |
| Vessel dilation, n (%) | 8 (1.42%) | 4 (1.10%) | 4 (2%) | .349 |
| Two stent technique during index PCI, n (%) | 12 (2.13%) | 8 (2.20%) | 4 (2%) | .912 |
| Aspiration during procedure, n (%) | 36 (6.38%) | 28 (4.96%) | 8 (4%) | .912 |
| Stent type during index PCI | ||||
| SES, n (%) | 442 (78.37%) | 274 (75.27%) | 168 (84%) | .134 |
| ZES, n (%) | 62 (11.00%) | 46 (12.64%) | 16 (8%) | .234 |
| EVS, n (%) | 62 (11.00%) | 44 (12.09%) | 18 (9%) | .428 |
| Stent overlap, n (%) | 178 (31.56%) | 108 (29.67%) | 70 (35%) | .357 |
| Post-dilation, n (%) | 126 (22.34%) | 86 (23.63%) | 40 (20%) | .357 |
| Minimum stent diameter (mm) | 2.89 ± 0.37 | 2.91 ± 0.38 | 2.84 ± 0.33 | .130 |
| Maximum stent diameter (mm) | 2.99 ± 0.37 | 3.03 ± 0.38 | 2.93 ± 0.33 | .042 |
| Total stent length (mm) | 39.63 ± 19.47 | 38.62 ± 20.04 | 41.47 ± 18.34 | .026 |
| Release pressure (atm) | 13.46 ± 2.73 | 13.62 ± 2.88 | 13.16 ± 2.42 | .171 |
| No. of stents per artery | 1.42 ± 0.64 | 1.40 ± 0.64 | 1.44 ± 0.66 | .579 |
| Parameters during PCI for VLST | ||||
| Spontaneous recanalization after AMI, n (%) | 228 (40.43%) | 146 (40.11%) | 82 (41%) | <0.001 |
| Aspiration during procedure, n (%) | 102 (18.09%) | 60 (16.48%) | 42 (21%) | .884 |
| Revascularization therapy | ||||
| Angioplasty only, n (%) | 212 (37.59%) | 126 (34.62%) | 86 (43%) | .346 |
| Re-stenting, n (%) | 336 (59.57%) | 224 (61.54%) | 118 (54%) | .218 |
| CABG, n (%) | 20 (3.55%) | 14 (3.85%) | 6 (3%) | .975 |
| Stent type during re-stenting | ||||
| SES (n = 334, 226, 108 in each group) | 208 (62.28%) | 134 (59.29%) | 74 (68.52%) | .713 |
| ZES (n = 334, 226, 108 in each group) | 80 (23.95%) | 60 (26.55%) | 20 (18.52%) | .250 |
| EVS (n = 334, 226, 108 in each group) | 46 (13.77%) | 32 (14.16%) | 14 (12.96%) | .255 |
AMI, acute myocardial infarction; CABG, coronary artery bypass graft; CABG, coronary artery bypass graft; EES, everolimus eluting stent; LAD, left anterior descending artery; LCX, left circumflex artery; PCI, percutaneous coronary intervention; RCA, right coronary artery; PCI, percutaneous coronary intervention; SES, sirolimus eluting stent; ZES, zotarolimus eluting stent.
Parameters of Emergent PCI for VLST
The parameters for emergent PCI for VLST are listed in Table 2. Spontaneous reperfusion occurred in 228 patients (40.43%), and thrombus aspiration was performed in 102 (18.09%) patients. Of these, 184 patients (32.62%) were treated with balloon angioplasty only, 24 patients (4.26%) were treated with drug coated balloons after balloon angioplasty, 332 patients (58.87%) underwent balloon angioplasty followed by implantation of an additional stent, and 20 patients (3.55%) underwent CABG after urgent coronary angiography.
Outcomes of Patients with VLST
After a median follow-up of 620 (249–1281) days, MACE occurred in 200 (35.46%) patients. Sixteen patients (2.8%) died of a cardiac cause during hospitalization. At the longest available follow-up, 24 of 40 deaths (60%) were cardiac in nature. The cumulative incidence of all-cause mortality and cardiac mortality was 3.7% and 2.1%, respectively, at 1 year; 7.2% and 5.1%, respectively, at 2 years; and 12.1% and 8.4%, respectively at 3 years. During the follow-up, heart failure requiring hospitalization occurred in 122 (21.63%) patients, 86 of whom (70.5%) had MI twice, and 70 (57.4%) of whom had LAD lesions. The cumulative incidence of heart failure requiring hospitalization at 1, 2, and 3 years was 15.6%, 20.7%, and 27.1%, respectively. A total of 44 (7.8%) cases of angiographically confirmed recurrent stent thrombosis were observed. The timing of definite recurrent stent thrombosis was subacute in two patients, late in 18 patients, and very late in 24 patients. The cumulative incidence of definite recurrent stent thrombosis at 30 days, and 1, 2, and 3 years was 0.4%, 3.8%, 6.8%, and 11.3%, respectively. Of note, recurrent stent thrombosis was the most common cause of MI (84%) during follow up. At the longest follow-up, TVR (including emergent PCI for recurrent stent thrombosis) was performed in 64 (11.35%) patients: 62 (96.88%) with repeated PCI and 2 (3.12%) with CABG. Twenty-four (37.5%) TVRs were associated with angioplasty only, 6 (9.38%) were associated with balloon angioplasty followed by drug coated balloons, and 32 (50%) were associated with additional DES implantation.
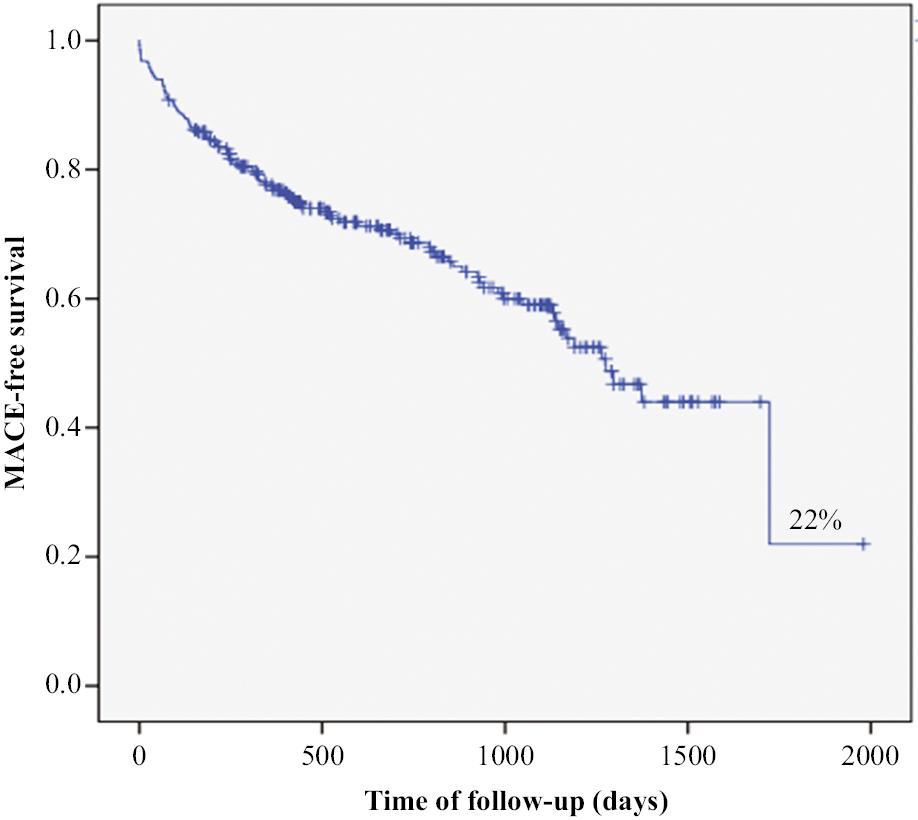
At the longest available follow-up, continuous DAPT had been administered to 374 (68.25%) patients. In the remaining patients, aspirin or clopidogrel was taken at least 1 year after the occurrence of VLST, but no significant difference was observed between patients with versus without MACE. Moreover, no significant difference was found among revascularization strategies (angioplasty alone, 37.59%, P = 0.346; re-stent, 58.87%, P = 0.218; CABG, 3.55%, P = 0.975). Kaplan-Meier survival analysis indicated that the estimated MACE free survival during follow-up was only 22% (Figure 1). After adjustment in the Cox proportional hazard model, peak troponin I and left ventricular ejection fraction (LVEF) were found to be independent predictors of MACE (Table 3 and Supplementary Table S1).
Multivariable Cox Regression Analysis of the Predictors of MACE.
| Variables | HR | 95%CI | P value |
|---|---|---|---|
| Age | 1.012 | 0.989–1.012 | .302 |
| Killip ≥2 | 1.675 | 0.999–2.801 | .051 |
| Peak troponin I | 1.003 | 1.001–1.005 | .015 |
| WBC | 1.029 | 0.967–1.095 | .369 |
| LVEF | 0.960 | 0.940–0.981 | <0.001 |
| eGFR | 0.995 | 0.985–1.005 | .337 |
eGFR, estimated glomerular filtration rate; LVEF, left ventricular eject fraction; WBC, white blood cell.
Discussion
This observational study in a large cohort of patients with definite VLST demonstrated that the clinical outcomes after revascularization remained unfavorable despite improvements in various revascularization strategies. Peak troponin I and LVEF were independent predictors of MACE in this group of patients.
The rates of in-hospital mortality and long-term mortality observed in patients with VLST were similar to those previously reported (3% and 10%, respectively) [7–10]; hence, our findings suggested that no improvement was achieved in mortality during the past decade. The high incidence of heart failure was remarkable. More than one-fifth of patients had heart failure requiring hospitalization during the follow-up. Prior studies on the prognosis of patients with VLST have rarely paid substantial attention to readmission for heart failure [7, 8], although this complication is not rare among patients with VLST. Possible explanations for the high incidence of heart failure may include the following: first, patients who receive PCI for MI might be likely to develop stent thrombosis [11]; second, most patients with VLST have acute MI [12]; third, stented LADs are likely to develop VLST [13]. Currently, recurrent stent thrombosis and TVR have not been completely eradicated. In this study, the incidence of both events was lower than previously reported [7, 8], possibly because of progress in PCI technology and the application of more effective antiplatelet agents in recent years.
Given the observational nature of the present study, the best available treatment for VLST could not be determined. Previous observational studies have reported varying results regarding the effects of angioplasty alone and re-stenting in patients with stent thrombosis [7, 10, 13, 14]. In this study, no significant difference in long-term outcomes was observed among revascularization strategies. Of note, intravascular ultrasound or optical coherence tomography may provide new insights into the treatment of patients with VLST [9], and randomized controlled trials are urgently needed to determine the optimal revascularization strategy for this group of patients. Moreover, controversy regarding the duration of DAPT after DES implantation persists. Previous studies have shown that prolonged DAPT may decrease ischemic events but increase bleeding events, without changing the overall prognosis [15]. Studies in patients with VLST have indicated that extending the duration of DAPT might improve overall prognosis [7]. However, in this study, all patients received standard antiplatelet therapy for more than 1 year after PCI. More than half the patients received aspirin at the onset of VLST, and nearly one-third of the patients received DAPT, yet VLST nonetheless occurred. Moreover, long-term antiplatelet therapy was not found to decrease the incidence of MACE in this study. The DAPT guidelines released in 2017 recommended shortening the duration of DAPT according to the DAPT risk score [16]. Hence, individualized anti-platelet therapy based on patient characteristics and the results of intravascular imaging might be favorable. Additionally, although univariable Cox regression analysis indicated that age, Killip ≥2, peak troponin I, WBC count, LVEF, and eGFR were possible predictors of MACE after VLST in this study, multivariable Cox regression analysis confirmed that the independent risk factors affecting the prognosis were LVEF and peak troponin I, thus potentially indicating diminished heart function after recurrence of myocardial infarction. Therefore, VLST prevention might be more important than treatment. Identifying potential high-risk patients with VLST and providing active intervention as early as possible might be a valuable strategy to avoid VLST. However, determining the pathogenesis underlying VLST is much more important. We have obtained serum samples from patients before and after the initial PCI, and hope to find additional clues regarding VLST pathogenesis.
This study had several limitations. First, this study was an observational study, and the sample size was insufficient to allow for more specific subgroup analysis; second, because few patients underwent intravascular imaging, the effects of such imaging on prognosis were not determined; third, the observational nature of the present study did not enable the effects of different revascularization strategies on prognosis to be assessed.