- Record: found
- Abstract: found
- Article: found
Posthematopoietic stem cell transplant COVID‐19 infection in a pediatric patient with IPEX syndrome
letter
Minelys M. Alicea Marrero
1
,
,
Margarita Silio
2 ,
Katie McQueen‐Amaker
1 ,
María Español
1 ,
María Velez
1 ,
Zachary LeBlanc
1
23 September 2020
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
To the Editor:
December 2019 marked the emergence of the novel severe acute respiratory syndrome
coronavirus 2 (SARS‐CoV‐2).
8
,
9
Several treatment approaches are under study. The antiviral remdesivir
10
has been shown to improve overall mortality in patients treated for COVID‐19,
1
and was approved by the United States Food and Drug Administration (FDA) for hospitalized
patients with severe disease.
2
Tocilizumab, a humanized antiinterleukin‐6 receptor
11
antibody, can hasten COVID‐19‐related cytokine release syndrome recovery by 75%.
3
Implementation of COVID‐19 convalescent plasma (CCP) in the treatment of COVID‐19
infection was also suggested by the FDA.
4
An 8‐year‐old African‐American male with immune‐dysregulation polyendocrinopathy X‐linked
(IPEX) syndrome underwent haploidentical, related bone marrow hematopoietic stem cell
transplant (HSCT), and contracted SARS‐CoV‐2 during the periengraftment period, subsequently
developing primary graft failure. The conditioning regimen included busulfan, fludarabine,
rabbit antithymoglobulin, and posttransplant cyclophosphamide; graft versus host disease
(GVHD) prophylaxis consisted of mycophenolate mofetil and cyclosporine.
Lack of engraftment and fever were noted on Day + 21 posttransplant. A sedated bone
marrow aspiration was planned; prior to sedation he tested positive for COVID‐19 via
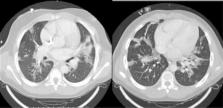
nucleic acid amplification test. Development of respiratory distress prompted a chest
CT that showed “bilateral ground‐glass opacities” (Figure 1); noninvasive ventilation
was initiated. A 10‐day‐course treatment with remdesivir began on Day + 26.
FIGURE 1
Chest computer tomography showing “ground‐glass” opacities consistent with COVID‐19
infection
We trended inflammatory parameters daily (Table 1), and based our treatment decision
on a calculated H‐score
5
of 209, which correlated with a 92.8% risk probability of cytokine release syndrome.
Two doses of tocilizumab and one unit of CCP
12
were given. On Day + 32, severe hypotension, acute hypoxemia, and mildly increased
right ventricle systolic pressure ensued, requiring mechanical ventilation and nitric
oxide. A comprehensive evaluation for superimposed infections was remarkable for a
repeated positive SARS‐CoV‐2 test, Staphylococcus epidermidis and Candida parapsilosis
infections, and BK and cytomegalovirus viremias.
TABLE 1
Inflammatory markers trend after starting treatment for COVID‐19 infection
Inflammatory markers
COVID treatment day
Days posttransplant
Ferritin (ng/mL)
Procalcitonin (ng/mL)
LDH (U/L)
CRP (mg/dL)
D‐dimer (ug/mL FEU)
Day −2
24
7790
0.5
432
9.87
13.4
Day −1
25
14 167
0.68
626
10.8
18.31
Start of treatment
[Link]
,
[Link]
26
14 272
1.04
780
11.8
17.11
Day 2
c
27
13 619
2.39
1019
16.2
16.5
Day 3
d
28
13 871
1.65
1092
8.9
13.6
Day 4
29
10 532
1.18
1021
4.2
11.62
Day 5
30
8797
0.66
962
2.3
9.67
Day 6
31
8496
0.51
1018
1.7
10.62
Day 7
32
6594
0.33
827
1.1
8.9
Day 8
33
6533
0.18
857
0.7
8.45
Day 9
34
5153
0.45
719
1.1
12.99
Day 10
e
35
4971
0.7
629
4.5
10.55
Day 11
36
6066
0.57
645
4
5.96
Day 12
37
5145
0.64
801
5.2
8.13
Day 13
38
4648
0.69
945
5
8.39
Day 14
39
6588
0.85
1208
4.2
7.92
Day 15
40
8727
0.81
1140
3
7.09
Day 16
f,g
41
10 294
1.5
1136
2.3
8.01
Day 17
42
28 884
2.99
1093
2.2
9.33
Note. Highest values are highlighted for each inflammatory marker.
a
First dose of remdesivir.
bFirst dose of tocilizumab.
c
Second dose of tocilizumab.
d
First convalescent plasma transfusion.
e
Treatment with remdesivir completed.
fThird dose of tocilizumab.
gSecond convalescent plasma transfusion.
John Wiley & Sons, Ltd.
This article is being made freely available through PubMed Central as part of the
COVID-19 public health emergency response. It can be used for unrestricted research
re-use and analysis in any form or by any means with acknowledgement of the original
source, for the duration of the public health emergency.
Bone marrow aplasia, lack of donor marrow CD33+ cells, absence of donor‐specific antibodies,
and compatible forward and backward flow cytometric crossmatches confirmed primary
graft failure and immune rejection, commonly seen in patients with IPEX syndrome.
In preparation for a second haploidentical related CD34+ selected peripheral hematopoietic
stem cell infusion, conditioning with fludarabine for 3 days began on Day + 39 posttransplant.
Salvage therapy with a second unit of CCP and a third dose of tocilizumab was given
on Day + 41. However, despite all efforts, he died on Day + 42 posttransplant.
Compared to their immunocompetent counterparts, immunocompromised patients with COVID‐19
are at increased risk for secondary infections and progression to severe disease,
as well as a different response to supportive care measures.
6
Studies have shown that SARS‐CoV‐2 acts mainly on T‐lymphocytes; hence, a severely
immunocompromised patient experiences a poorer outcome. A case report depicted two
adult posttransplant patients with adequate graft function, on immunosuppressive therapy,
that eventually died after developing multiorgan failure.
7
Our patient was treated aggressively, and we attributed the first decreasing trend
in inflammatory markers (Table 1) to achieving disease control. However, the combination
of graft failure, COVID‐19 infection with multiorgan failure, and opportunistic infections
contributed to his death. We hope that new treatments continue to stem from ongoing
research, to achieve a different outcome in our patient population.
Related collections
Most cited references9
- Record: found
- Abstract: found
- Article: found
COVID-19: consider cytokine storm syndromes and immunosuppression
Puja Mehta, Daniel McAuley, Michael Brown … (2020)
- Record: found
- Abstract: found
- Article: not found
Effect of Convalescent Plasma Therapy on Viral Shedding and Survival in COVID-19 Patients
Qing-Lei Zeng, Zu-Jiang Yu, Jian-Jun Gou … (2020)
