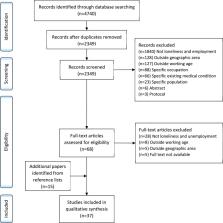
Introduction “Social relationships, or the relative lack thereof, constitute a major risk factor for health—rivaling the effect of well established health risk factors such as cigarette smoking, blood pressure, blood lipids, obesity and physical activity” —House, Landis, and Umberson; Science 1988 [1] Two decades ago a causal association between social relationships and mortality was proposed after a review of five large prospective studies concluded that social relationships predict mortality [1]. Following the publication of this provocative review, the number of prospective studies of mortality that included measures of social relationships increased exponentially. Although the inverse association between social relationships and nonsuicide mortality has received increased attention in research, neither major health organizations nor the general public recognize it as a risk factor for mortality. This may be due in part to the fact that the literature has become unwieldy, with wide variation in how social relationships are measured across a large number of studies and disappointing clinical trials [2]. “Social relationships” has perhaps become viewed as a fuzzy variable, lacking the level of precision and control that is preferred in biomedical research. Thus, the large corpus of relevant empirical research is in need of synthesis and refinement. Current evidence also indicates that the quantity and/or quality of social relationships in industrialized societies are decreasing. For instance, trends reveal reduced intergenerational living, greater social mobility, delayed marriage, dual-career families, increased single-residence households, and increased age-related disabilities [3],[4]. More specifically, over the last two decades there has been a three-fold increase in the number of Americans who report having no confidant—now the modal response [3]. Such findings suggest that despite increases in technology and globalization that would presumably foster social connections, people are becoming increasingly more socially isolated. Given these trends, understanding the nature and extent of the association between social relationships and mortality is of increased temporal importance. There are two general theoretical models that propose processes through which social relationships may influence health: the stress buffering and main effects models [5]. The buffering hypothesis suggests that social relationships may provide resources (informational, emotional, or tangible) that promote adaptive behavioral or neuroendocrine responses to acute or chronic stressors (e.g., illness, life events, life transitions). The aid from social relationships thereby moderates or buffers the deleterious influence of stressors on health. From this perspective, the term social support is used to refer to the real or perceived availability of social resources [6]. The main effects model proposes that social relationships may be associated with protective health effects through more direct means, such as cognitive, emotional, behavioral, and biological influences that are not explicitly intended as help or support. For instance, social relationships may directly encourage or indirectly model healthy behaviors; thus, being part of a social network is typically associated with conformity to social norms relevant to health and self-care. In addition, being part of a social network gives individuals meaningful roles that provide self-esteem and purpose to life [7],[8]. Social relationships have been defined and measured in diverse ways across studies. Despite striking differences, three major components of social relationships are consistently evaluated [5]: (a) the degree of integration in social networks [9], (b) the social interactions that are intended to be supportive (i.e., received social support), and (c) the beliefs and perceptions of support availability held by the individual (i.e., perceived social support). The first subconstruct represents the structural aspects of social relationships and the latter two represent the functional aspects. Notably, these different subconstructs are only moderately intercorrelated, typically ranging between r = 0.20 and 0.30 [9],[10]. While all three components have been shown to be associated with morbidity and mortality, it is thought that each may influence health in different ways [11],[12]. Because it is presently unclear whether any single aspect of social relationships is more predictive than others, synthesis of data across studies using several types of measures of social relationships would allow for essential comparisons that have not been conducted on such a large scale. Empirical data suggest the medical relevance of social relationships in improving patient care [13], increasing compliance with medical regimens [13], and promoting decreased length of hospitalization [14],[15]. Likewise, social relationships have been linked to the development [16],[17] and progression [18]–[21] of cardiovascular disease [22]—a leading cause of death globally. Therefore, synthesis of the current empirical evidence linking social relationships and mortality, along with clarifications of potential moderators, may be particularly relevant to public health and clinical practice for informing interventions and policies aimed at reducing risk for mortality. To address these issues, we conducted a meta-analysis of the literature investigating the association between social relationships and mortality. Specifically, we addressed the following questions: What is the overall magnitude of the association between social relationships and mortality across research studies? Do structural versus functional aspects of social relationships differentially impact the risk for mortality? Is the association moderated by participant characteristics (age, gender, health status, cause of mortality) or by study characteristics (length of clinical follow-up, inclusion of statistical controls)? Is the influence of social relationships on mortality a gradient or threshold effect? Methods Identification of Studies To identify published and unpublished studies of the association between social relationships and mortality, we used three techniques. First, we conducted searches of studies from January 1900 to January 2007 using several electronic databases: Dissertation Abstracts, HealthSTAR, Medline, Mental Health Abstracts, PsycINFO, Social Sciences Abstracts, Sociological Abstracts via SocioFile, Academic Search Premier, ERIC, and Family & Society Studies Worldwide. To capture the broadest possible sample of relevant articles, we used multiple search terms, including mortality, death, decease(d), died, dead, and remain(ed) alive, which were crossed with search words related to social relationships, including the terms social and interpersonal linked to the following words: support, network, integration, participation, cohesion, relationship, capital, and isolation To reduce inadvertent omissions, we searched databases yielding the most citations (Medline, PsycINFO) two additional times. Next, we manually examined the reference sections of past reviews and of studies meeting the inclusion criteria to locate articles not identified in the database searches. Finally, we sent solicitation letters to authors who had published three or more articles on the topic. Inclusion Criteria We included in the meta-analysis studies that provided quantitative data regarding individuals' mortality as a function of social relationships, including both structural and functional aspects [23]. Because we were interested in the impact of social relationships on disease, we excluded studies in which mortality was a result of suicide or injury. We also excluded studies in which the only measurement of social support was an intervention provided within the context of the study (e.g., support group), the source of social support was nonhuman (e.g., a pet or higher power), or the social support was provided to others (i.e., giving support to others or measures of others' benefit from the support provided) rather than to the individual tracked for mortality status. We coded studies that included participant marital status as one of several indicators of social support, but we excluded studies in which marital status was the only indicator of social support. We also excluded studies in which the outcome was not explicitly and solely mortality (e.g., combined outcomes of morbidity/mortality). Reports with exclusively aggregated data (e.g., census-level statistics) were also excluded. Manuscripts coded were all written in English, which accounted for 98% of the total retrieved. See Figure 1 for additional details. 10.1371/journal.pmed.1000316.g001 Figure 1 Flow diagram. Data Abstraction To increase the accuracy of coding and data entry, each article was initially coded by two raters. Subsequently, the same article was independently coded by two additional raters. Coders extracted several objectively verifiable characteristics of the studies: (a) the number of participants and their composition by age, gender, marital status, distress level, health status, and pre-existing health conditions (if any), as well as the percentage of smokers and percentage of physically active individuals, and, of course, the cause of mortality; (b) the length of follow up; (c) the research design; and (d) the aspect of social relationships evaluated. Data within studies were often reported in terms of odds ratios (ORs), the likelihood of mortality across distinct levels of social relationships. Because OR values cannot be meaningfully aggregated, all effect sizes reported within studies were transformed to the natural log OR (lnOR) for analyses and then transformed back to OR for interpretation. When effect size data were reported in any metric other than OR or lnOR, we transformed those values using statistical software programs and macros (e.g., Comprehensive Meta-Analysis [24]). In some cases when direct statistical transformation proved impossible, we calculated the corresponding effect sizes from frequency data in matrices of mortality status by social relationship status. When frequency data were not reported, we recovered the cell probabilities from the reported ratio and marginal probabilities. When survival analyses (i.e., hazard ratios) were reported, we calculated the effect size from the associated level of statistical significance, often derived from 95% confidence intervals (CIs). Across all studies we assigned OR values less than 1.00 to data indicative of increased mortality and OR values greater than 1.00 to data indicative of decreased mortality for individuals with relatively higher levels of social relationships. When multiple effect sizes were reported within a study at the same point in time (e.g., across different measures of social relationships), we averaged the several values (weighted by standard error) to avoid violating the assumption of independent samples. In such cases, the aggregate standard error value for the lnOR were estimated on the basis of the total frequency data without adjustment for possible correlation among the averaged values. Although this method was imprecise, the manuscripts included in the meta-analysis did not report the information necessary to make the statistical adjustments, and we decided not to impute values given the wide range possible. In analyzing the data we used the shifting units of analysis approach [25] which minimizes the threat of nonindependence in the data while at the same time allowing more detailed follow-up analyses to be conducted (i.e., examination of effect size heterogeneity). When multiple reports contained data from the same participants (publications of the same database), we selected the report containing the whole sample and eliminated reports of subsamples. When multiple reports contained the same whole sample, we selected the one with the longest follow-up duration. When multiple reports with the same whole sample were of the same duration, we selected the one reporting the greatest number of measures of social relationships. In cases where multiple effect sizes were reported across different levels of social relationships (i.e., high versus medium, medium versus low), we extracted the value with the greatest contrast (i.e., high versus low). When a study contained multiple effect sizes across time, we extracted the data from the longest follow-up period. If a study used statistical controls in calculating an effect size, we extracted the data from the model utilizing the fewest statistical controls so as to remain as consistent as possible across studies (and we recorded the type and number of covariates used within each study to run post hoc comparative analyses). We coded the research design used rather than estimate risk of individual study bias. The coding protocol is available from the authors. The majority of information obtained from the studies was extracted verbatim from the reports. As a result, the inter-rater agreement was quite high for categorical variables (mean Cohen's kappa = 0.73, SD = 0.13) and for continuous variables (mean intraclass correlation [26] = 0.80, SD = .14). Discrepancies across coding pairs were resolved through further scrutiny of the manuscript until consensus was obtained. Aggregate effect sizes were calculated using random effects models following confirmation of heterogeneity. A random effects approach produces results that generalize beyond the sample of studies actually reviewed [27]. The assumptions made in this meta-analysis clearly warrant this method: The belief that certain variables serve as moderators of the observed association between social relationships and mortality implies that the studies reviewed will estimate different population effect sizes. Random effects models take such between-studies variation into account, whereas fixed effects models do not [28]. In each analysis conducted, we examined the remaining variance to confirm that random effects models were appropriate. Results Statistically nonredundant effect sizes were extracted from 148 studies ([29]–[176]; see Table 1). Data were reported from 308,849 participants, with 51% from North America, 37% from Europe, 11% from Asia, and 1% from Australia. Across all studies, the average age of participants at initial evaluation was 63.9 years, and participants were evenly represented across sex (49% female, 51% male). Of the studies examined, 60% involved community samples, but 24% examined individuals receiving outpatient medical treatment, and 16% utilized patients in inpatient medical settings. Of studies involving patients with a pre-existing diagnosis, 44% were specific to cardiovascular disease (CVD), 36% to cancer, 9% to renal disease, and the remaining 11% had a variety of conditions including neurological disease. Research reports most often (81%) considered all-cause mortality, but some restricted evaluations to mortality associated with cancer (9%), CVD (8%), or other causes (2%). Participants were followed for an average of 7.5 years (SD = 7.1, range = 3 months to 58 years), with an average of 29% of the participants dying within each study's follow-up period. 10.1371/journal.pmed.1000316.t001 Table 1 Overview of the 148 studies included in the meta-analysis. Source Total Number of Participants Average Age at Intake Location of Study Study Length Cause of Mortality Social Relationship Measure Original Statistic Metric lnOR Standard Error Ahern et al., 1990 [29] 353 50 USA 1 y All-cause Functional M & SD 0.27 0.36 Alter et al., 2006 [30] 3,138 64 Canada 5 y 4 m CVD Combined Chi 0.06 0.15 Anstey et al., 2002 [31] 2,065 78 Australia 9 y All-cause Structural Freq 0.44 0.09 Astrand et al., 1989 [32] 391 50 Sweden 22 y All-cause Combined OR 0.00 0.18 Avlund et al., 1998 [33] 727 70 Denmark 11 y All-cause Combined OR 0.40 0.16 Avlund et al., 2004 [34] 565 75 Denmark, Finland 5 y All-cause Structural OR 0.54 0.22 Barefoot et al., 2005 [35] 3,109 58 Denmark 7 y 2 m All-cause Structural p 0.15 0.12 Berkman and Syme, 1979 [36] 4,765 47 USA 9 y All-cause Structural Freq 0.60 0.30 Berkman et al., 2004 [37] 3,495 45 France 10 y All-cause Structural RR 1.61 0.14 Birket-Smith et al., 1989 [38] 128 73 Denmark 1 y All-cause Structural R 0.37 0.33 Blazer, 1982 [39] 331 72 USA 2 y 6 m All-cause Combined RR 1.05 0.30 Blazer et al., 2001 [40] 3,664 73 USA 3 y All-cause Combined OR 0.15 0.10 Bowling, 1989 [41] 503 73 UK 6 y All-cause Structural Chi 0.51 0.16 Brown et al., 2003 [42] 846 NR USA 5 y All-cause Combined OR 0.01 0.22 Brummet et al., 2005 [43] 2,711 62 USA 11 y 1m All-cause Functional p 0.25 0.17 Burg et al., 2005 [44] 1,899 75 USA 2 y 5 m All-cause Combined Freq 1.39 0.28 Burns et al., 2005 [45] 147 63 Australia 7 y 4 m Cancer Combined Combin 0.45 0.31 Butow et al., 1999 [46] 125 55 Australia 2 y Cancer Combined p 0.35 0.33 Bygren et al., 1996 [47] 12,675 43 Sweden 9 y All-cause Structural Freq 0.41 0.07 Case et al., 1992 [48] 1,195 59 Canada, USA 4 y 2 m CVD Structural RR 0.68 0.25 Cassileth et al., 1988 [49] 203 60 USA 8 y Cancer Structural Combin −0.03 0.26 Ceria et al., 2001 [50] 1,786 78 USA 6 y All-cause Structural RR 1.01 0.12 Chacko et al., 1996 [51] 94 53 USA 4 y 8 m CVD Functional Chi 0.92 0.39 Christensen et al., 1999 [52] 133 29 USA 58 y 2m All-cause Combined Chi 0.98 0.32 Christensen et al., 1994 [53] 78 54 USA 5 y All-cause Functional Chi 0.98 0.44 Cohen et al., 1987 [54] 155 73 USA 3 y All-cause Structural T 0.65 0.30 Colon et al., 1991 [55] 100 30 USA 2 y Cancer Functional Chi 0.86 0.38 Cornman et al., 2003 [56] 4,049 NR Taiwan 3 y All-cause Structural OR 0.17 0.06 Coyne et al., 2001 [57] 189 53 USA 4 y CVD Functional RR 0.99 0.26 Cree et al., 2000 [58] 558 82 Canada 4 m All-cause Functional OR 0.30 0.34 Cuijpers, 2001 [59] 424 85 Netherlands 1 y All-cause Functional OR −0.10 0.31 Dalgard & Haheim, 1998 [60] 1,002 46 Norway 17 y All-cause Structural p 0.23 0.15 Devins et al., 1990 [61] 97 40 Canada 4 y Other Structural R −0.025 0.38 Dickens et al., 2004 [62] 556 60 UK 1 y CVD Functional p 0.65 0.45 Ell et al., 1992 [63] 294 61 USA 6 y 11m All-cause Combined p −0.15 0.21 Eng et al., 2002 [64] 16,242 55 USA 10 y All-cause Structural RR 0.42 0.06 Engedal,1996 [65] 334 82 Norway 3 y All-cause Structural M & SD 0.62 0.20 Farmer et al., 1996 [66] 320 60 USA 4 y 7m All-cause Combined RR 0.81 0.22 Forster & Stoller, 1992 [67] 363 74 USA 7 y All-cause Combined LnOR −0.20 0.22 Frasure-Smith et al., 2000 [68] 887 59 Canada 1 y CVD Functional p 0.09 0.12 Frick et al., 2005 [69] 99 55 Germany 3 y 11m Cancer Combined p 0.23 0.35 Fry and Debats, 2006 [70] 380 75 Canada 5 y 11m All-cause Combined RR 0.78 0.24 Fuhrer et al., 1999 [71] 3,777 76 France 5 y All-cause Combined RR 0.38 0.13 Funch & Marshall, 1983 [72] 208 51 USA 20 y Cancer Structural Combin 0.17 0.26 Ganzini et al., 1997 [73] 100 73 USA 2 y 6m All-cause Combined Combin 0.15 0.25 Gellert et al., 1993 [74] 136 47 USA 10 y Cancer Functional RR −0.24 0.40 Giles et al., 2005 [75] 1,477 80 Australia 10 y All-cause Structural p 0.21 0.10 Giraldi et al., 1997 [76] 74 51 Italy 6 y Cancer Functional M & SD 0.14 0.43 Glass et al., 1999 [77] 1,380 72 USA 13 y All-cause Structural RR 0.42 0.20 Goldman et al., 1995 [78] 7,478 77 USA 6 y All-cause Structural OR 0.30 0.06 Goodwin et al., 1996 [79] 328 72 USA 10 y All-cause Structural p 0.62 0.20 Gorkin et al.,1993 [80] 1,146 61 USA 10 m All-cause Functional Freq 0.23 0.28 Grand et al., 1990 [81] 645 75 France 4 y All-cause Combined OR 0.40 0.22 Greenfield et al., 2002 [82] 5,092 NR USA 11 y All-cause Structural RR 0.38 0.14 Greenwood et al., 1995 [83] 1,274 59 UK 4 y All-cause Structural RR 0.43 0.17 Grodner et al., 1996 [84] 110 63 USA 6 y All-cause Combined M & SD 0.50 0.35 Gustafsson et al., 1998 [85] 421 81 Sweden 6 y All-cause Structural OR 0.24 0.19 Hall et al., 1993 [86] 5,921 60 Sweden 11 y CVD Structural OR 0.23 0.15 Helweg-Larsen, 2003 [87] 6,617 44 Denmark 13 y All-cause Combined RR 0.74 0.05 Herndon et al., 1999 [88] 206 61 USA 4 y 2 m Cancer Functional p 0.16 0.26 Hill et al., 2005 [89] 3,050 78 USA 8 y All-cause Combined p 0.08 0.07 Hirdes & Forbes, 1992 [90] 259 45 Canada 20 y All-cause Combined RR 0.55 0.29 Ho, 1991 [91] 946 77 China 2 y All-cause Combined RR 0.55 0.24 House et al., 1982 [92] 2,754 52 USA 12 y All-cause Structural Combin 0.27 0.17 Hummer et al., 1999 [93] 21,204 43 USA 8 y All-cause Structural Freq 0.45 0.05 Iribarren et al., 2005 [94] 5,108 25 USA 16 y All-cause Structural Combin 0.60 0.21 Irvine et al., 1999 [95] 634 64 Canada 2 y All-cause Structural RR 0.01 0.32 Iwasaki et al., 2002 [96] 11,560 55 Japan 7 y All-cause Combined RR 0.22 0.11 Johnson et al., 2005 [97] 3,698 43 USA 5 y All-cause Combined p 0.18 0.10 Johnson et al., 1996 [98] 1,257 64 Sweden 14 y CVD Functional RR 0.21 0.15 Jorm et al., 1991 [99] 228 79 Australia 5 y All-cause Functional M & SD 0.24 0.24 Juon et al., 2003 [100] 1,091 6 USA 28 y All-cause Structural OR 0.60 0.35 Jylhä and Aro, 1989 [101] 936 NR Finland 6 y 6 m All-cause Combined p 0.32 0.12 Kaplan et al., 1988 [102] 5,320 49 Finland 5 y All-cause Structural OR 0.75 0.18 Kaplan et al., 1994 [103] 2,501 53 Finland 5 y 11m All-cause Combined RR 0.27 0.19 Kawachi et al., 1996 [104] 18,702 60 USA 4 y All-cause Structural RR 0.50 0.17 Keller et al., 2003 [105] 654 78 USA 10 y All-cause Structural p 0.53 0.14 Kiely et al., 2000 [106] 916 87 USA 4 y 6 m All-cause Structural p 0.23 0.12 Kimmel et al., 2000 [107] 174 54 USA 5 y All-cause Functional p 0.73 0.17 Korten et al., 1999 [108] 752 70 Australia 4 y All-cause Combined Combin 0.20 0.13 Krause, 1997 [109] 2,209 68 UK 11 y All-cause Combined OR −0.03 0.10 Krause, 2006 [110] 976 74 USA 3 y All-cause Combined OR 0 0.18 Kroenke et al., 2006 [111] 2,835 59 USA 12 y All-cause Structural RR 0.45 0.22 La Cour et al., 2005 [112] 734 70 Denmark 20 y All-cause Structural p 0.45 0.14 Lee & Rotheram-Borus, 2001 [113] 307 38 USA 2 y 4 m Other Functional p 0.54 0.21 Lehto et al., 2006 [114] 101 54 Finland 9 y Cancer Functional p 0.97 0.38 Lennartsson and Silverstein, 2001 [115] 463 82 Sweden 4 y All-cause Structural RR 0.40 0.17 Ljungquist et al., 1995 [116] 956 70 Sweden 10 y All-cause Combined OR 1.03 0.16 Lund et al., 2002 [117] 1,265 60 Denmark 8 y All-cause Structural p 0.37 0.16 Lund et al., 2000 [118] 894 79 Denmark 8 y All-cause Structural OR 0.30 0.21 Lyyra and Heikkinen, 2006 [119] 206 80 Finland 10 y All-cause Combined Combin 0.25 0.30 Maier & Smith, 1999 [120] 513 85 Germany 6 y All-cause Functional Combin 0.33 0.16 Malmstrom et al., 2001 [121] 22,236 47 Sweden 8 y All-cause Structural RR 0.30 0.07 McClellan et al., 1993 [122] 210 55 USA 1 y All-cause Functional M & SD 0.24 0.34 Merlo et al., 2000 [123] 491 68 Sweden 10 y All-cause Combined Freq 0.63 0.19 Mertens et al., 1996 [124] 1,869 62 USA 4 y All-cause Structural M & SD 0.56 0.08 Morris et al., 1993 [125] 91 60 USA 10 y All-cause Structural T 0.81 0.40 Murata et al., 2005 [126] 1,994 73 Japan 7 y 4 m All-cause Combined p 0.12 0.11 Murberg and Bru, 2001 [127] 119 66 Norway 2 y CVD Combined p 0.27 0.34 Musick et al., 2004 [128] 3,617 47 USA 7 y 6 m All-cause Combined R 0.17 0.06 Nakanishi and Tatara, 2000 [129] 1,285 74 Japan 5 y 6 m All-cause Structural p 0.26 0.10 Nordentoft et al., 1993 [130] 974 41 Denmark 10 y All-cause Structural p 0.42 0.12 Olsen et al., 1991 [131] 1,637 79 Denmark 15 y 6m All-cause Combined p 0.14 0.11 Oman and Reed, 1998 [132] 2,023 75 USA 5 y 7 m All-cause Structural P 0.20 0.11 Orrell et al., 2000 [133] 60 80 UK 3 y All-cause Combined p 0.62 0.48 Orth-Gomer and Johnson, 1987 [134] 17,433 49 Sweden 6 y ? Structural RR 1.31 0.07 Orth-Gomer and Unden, 1990 [135] 147 57 Sweden 10 y All-cause Structural T 0.86 0.40 Ostbye et al., 2006 [136] 4,012 77 USA 10 y All-cause Combined OR 0.54 0.09 Oxman et al., 1995 [137] 232 76 USA 6 m CVD Combined Combin 0.33 0.46 Parkerson and Gutman, 2000 [138] 103 63 USA 1 y All-cause Structural OR 1.65 0.58 Pennix et al., 1997 [139] 2,829 70 Netherlands 3 y All-cause Combined Freq 0.30 0.15 Rasulo et al., 2005 [140] 1,734 81 Denmark 6 y All-cause Structural p 0.11 0.09 Reuben et al., 1992 [141] 259 73 USA 4 y 3 m All-cause Combined R 0.52 0.22 Reynolds et al., 1994 [142] 1,011 53 USA 5 y Cancer Combined p 0.19 0.17 Rodriguez-Artalejo et al., 2006 [143] 251 77 Spain 7 m CVD Structural p 0.17 0.33 Rosengren et al., 1998 [144] 717 50 Sweden 12 y All-cause Combined Freq 0.64 0.28 Roy et al., 1996 [145] 547 80 USA 4 y All-cause Structural RR 0.76 0.15 Rozzini et al., 1991 [146] 1,201 73 Italy 3 y All-cause Structural Freq 0.94 0.20 Ruberman et al., 1984 [147] 2,320 50 USA 3 y All-cause Structural Chi 0.39 0.08 Rutledge et al., 2003 [148] 7,524 71 USA 6 y All-cause Combined RR 0.53 0.05 Rutledge et al., 2004 [149] 503 59 USA 2 y 4 m All-cause Combined M & SD 0.99 0.37 Saito-Nakaya et al., 2006 [150] 238 62 Japan 7 y 6 m All-cause Combined Freq −0.07 0.35 Schoenbach et al., 1986 [151] 791 55 USA 2 y All-cause Structural Freq 0.80 0.19 Seeman et al., 1993 [152] 1,420 74 USA 5 y All-cause Combined p 1.83 0.17 Shahatahmasebi et al., 1992 [153] 534 72 UK 8 y All-cause Combined Chi 0.40 0.16 Shmotkin et al., 2003 [154] 1,174 84 Israel 8 y All-cause Structural p −0.09 0.12 Shye et al., 1995 [155] 455 72 USA 15 y All-cause Structural Freq 0.80 0.21 Silverstein and Bengston, 1991 [156] 435 67 USA 14 y All-cause Combined OR 0.03 0.16 Soler-Vila et al., 2003 [157] 322 54 USA 10 y All-cause Combined M & SD 0.29 0.20 Stavraky et al., 1988 [158] 224 59 Canada 1 y Cancer Combined Freq 0.55 0.35 Stek et al., 2005 [159] 476 85 Netherlands 5 y All-cause Functional p 0.35 0.21 Sturdy et al., 2002 [160] 1,066 53 UK 5 y All-cause Structural OR 0.17 0.35 Sugisawa et al., 1994 [161] 1,943 69 Japan 3 y All-cause Combined p 0.03 0.19 Sun and Lui, 2006 [162] 7,938 92 China 2 y All-cause Structural R 0.67 0.04 Temkin-Greener et al., 2004 [163] 3,138 79 USA 2 y All-cause Combined p 0.21 0.10 Thomas et al., 1997 [164] 424 63 Canada, USA 3 y 11m CVD Functional M & SD 0.10 0.18 Tucker et al., 1996 [165] 1,077 12 USA 41 y All-cause Structural p 0.27 0.12 Vaillant et al., 1998 [166] 223 20 USA 25 y All-cause Combined OR 1.15 0.37 Vogt et al., 1992 [167] 2,396 47 USA 15 y All-cause Structural p 0.20 0.08 Walter-Ginzburg et al., 2002 [168] 1,340 83 Israel 8 y All-cause Combined Freq 0.23 0.11 Waxler-Morrison et al., 1991 [169] 118 45 Canada 4 y Cancer Structural p 0.27 0.36 Weihs et al., 2005 [170] 90 52 USA 9 y Cancer Structural Combin 0.61 0.40 Welin et al., 2000 [171] 275 55 Sweden 10 y All-cause Combined p 0.44 0.22 Welin et al., 1992 [172] 959 60 Sweden 12 y All-cause Combined Combin 0.52 0.17 Wilkins, 2003 [173] 2,107 75 Canada 6 y All-cause Combined RR 0.05 0.12 Woloshin et al., 1997 [174] 37 67 Canada 1 y All-cause Functional OR 1.87 0.61 Yasuda et al., 1997 [175] 806 74 USA 5 y All-cause Combined Freq 0.27 0.19 Zuckerman et al., 1984 [176] 398 72 USA 2 y All-cause Combined Combin 0.09 0.18 Chi, chi-square; Combin, combined statistics; Freq, frequency counts; m, months; M & SD, means and standard deviations; NR, not reported; OR, odds ratio; RR, risk ratio; p, level of statistical significance; t, t-scores; y, years. Omnibus Analysis Across 148 studies, the random effects weighted average effect size was OR = 1.50 (95% confidence interval [CI] = 1.42 to 1.59), which indicated a 50% increased likelihood of survival as a function of stronger social relations. Odds ratios ranged from 0.77 to 6.50, with substantial heterogeneity across studies (I2 = 81% [95% CI = 78% to 84%]; Q (147) = 790, p 0.05). Finally, we plotted a contour-enhanced funnel plot (Figure 2) [181]. The data obtained from this meta-analysis were fairly symmetrical with respect to their own mean; fewer than ten studies were “missing” on the left side of the distribution that would have made the plot symmetrical. Based on these several analyses, publication bias is unlikely to threaten the results. 10.1371/journal.pmed.1000316.g002 Figure 2 Contour enhanced funnel plot. Moderation by Social Relationship Assessment, and by Participant and Study Characteristics Given that structural versus functional components of social relationships may influence health in different ways [11],[12], the high degree of heterogeneity observed in the omnibus results may have been due in part to differences between the components of social relationships evaluated within and across studies. Hence the remaining analyses separately evaluate effect sizes obtained from structural, functional, and combined (structural and functional) measures of social relationships. Table 2 provides definitions of the types and subtypes of social relationships evaluated. 10.1371/journal.pmed.1000316.t002 Table 2 Descriptive coding of the measures used to assess social relationships. Type of Measure Description Example of Measure Functional Functions provided or perceived to be available by social relationships Received support Self-reported receipt of emotional, informational, tangible, or belonging support • Inventory of Social Supportive Behaviors [213]• UCLA Social Support Interview [214],[215]• Social Support Behaviors Scale [216] Perceptions of social support Perception of availability of emotional, informational, tangible, or belonging support if needed. • EPESE support questions [217]• Malmo Social Support Scale [218]• Social Support Questionnaire [219]• Interpersonal Support Evaluation List [220] Perception of loneliness Feelings of isolation, disconnectedness, and not belonging • Loneliness Scale [221]• UCLA Loneliness Scale [222] Structural The existence and interconnections among differing social ties and roles Marital status married versus other • Binary item: Married yes, no• Married, never married, divorced, separated, widowed Social networks network density or size, number of social contacts • Convoy measure [223]• Social Network List [224] Social integration Participation in a broad range of social relationships; including active engagement in a variety of social activities or relationships, and a sense of communality and identification with one's social roles. • Malmo Influence, Contact, & Anchorage Measure [225]• Social Network Index [226],[227]• Social Participation Scale [92] Complex measures of social integration A single measure that assesses multiple components of social integration such as marital status, network size and network participation. • Social Network Index [36]• Social Network Questionnaire [228]• Social Connections Index [102]• Rand Social Health Battery [229] Living alone Living alone versus living with others • Binary item: yes, no• Number of people in household Social isolation Pervasive lack of social contact or communication, participation in social activities, or confidant • Social Isolation Scale [82] Combined Assessment of both structural and functional measures Multifaceted Measurement Multiple measures obtained that assess more than one of the above conceptualizations. Structural aspects of social relationships Sixty-three studies had data exclusive to structural measures of social relationships (see Figure 3). Across these studies, the random effects weighted average effect size was OR = 1.57 (95% CI = 1.46 to 1.70), which value fell within the CI of the omnibus results reported previously. The heterogeneity across studies was still quite large (I2 = 84% [95% CI = 80% to 87%]; Q (62) = 390, p<0.001; τ2 = 0.07), so we undertook metaregression with prespecified participant and study characteristics. 10.1371/journal.pmed.1000316.g003 Figure 3 Forest plot of structural measures. Metaregression is an analogue to multiple regression analysis for effect sizes. Its primary purpose is to ascertain which continuous and categorical (dummy coded) variables predict variation in effect size estimates. Using random effects weighted metaregression, we examined the simultaneous association (with all variables entered into the model) between effect sizes and prespecified participant and study characteristics (Table 3). To examine the most precise effect size estimates available and to increase the statistical power associated with this analysis, we shifted the unit of analysis [24] and extracted effect sizes within studies that were specific to measures of structural aspects of social relationships. That is, if a study contained effect sizes from both structural and functional types of social relationships, we extracted the structural types for this analysis (with identical subtypes aggregated), which resulted in a total of 230 unique effect sizes across 116 studies. A total of 18% of the variance in these effect sizes was explained in the metaregression (p<0.001). As can be seen in Table 3, effect sizes based on data controlling for other variables were lower in magnitude than those based on raw data. Moreover, effect sizes differed in magnitude across the subtype of structural social relationships measured. Complex measures of social integration were associated with larger effect size values than measures of social participation. Binary measures of whether participants lived alone (yes/no) were associated with smaller effect size values. Average random effects weighted odds ratios for the various subtypes of social relationships are reported in Table 4. 10.1371/journal.pmed.1000316.t003 Table 3 Random effects metaregression for effect size estimates of structural social relationships. Variable B SE p β (Constant) 0.535 0.238 0.02 0.00 Participants' average agea −0.002 0.002 0.49 −0.06 Participant sex compositionb 100% Female 0.038 0.066 0.57 0.04 100% Male 0.049 0.068 0.48 0.05 Participant initial healthc −0.103 0.085 0.23 −0.10 Cause of mortalityd Cardiovascular disease 0.081 0.161 0.61 0.03 Cancer −0.208 0.139 0.13 −0.12 Length of follow-up evaluation (y) −0.003 0.005 0.54 −0.05 Measure of social relationshipse Living alone −0.265 0.106 0.013 −0.18 Marital status −0.097 0.074 0.19 −0.10 Social isolation −0.144 0.178 0.42 −0.05 Social networks −0.050 0.071 0.48 −0.06 Complex measures of integration 0.255 0.095 0.007 0.20 Geographic region of studyf Asia 0.057 0.154 0.71 0.05 Europe 0.221 0.134 0.10 0.25 North America 0.057 0.134 0.69 0.07 Statistically controlled estimateg −0.147 0.058 0.01 −0.17 a Age at study initiation. b Contrasted with reports in which males and females were combined. c Individuals with a pre-existing medical condition contrasted with community samples. d Contrasted with all cause and all other causes. e Contrasted with measures of social participation; see Table 2 for descriptions of each kind of measure. f Contrasted with all other world regions combined. g Contrasted with estimates based on raw data. β, standardized beta; B, unstandardized beta; SE, standard error. 10.1371/journal.pmed.1000316.t003 Table 4 Weighted average effect sizes across different measures of social relationships. Type of Measure k OR 95% CI Functional Received social support 9 1.22 [0.91, 1.63] Perceptions of social support 73 1.35 [1.22, 1.49] Loneliness (inversed) 8 1.45 [1.08, 1.94] Structural Living alone (inversed) 17 1.19 [0.99, 1.44] Marital status (married versus other) 62 1.33 [1.20, 1.48] Social isolation (inversed) 8 1.40 [1.06, 1.86] Social networks 71 1.45 [1.32, 1.59] Social integration 45 1.52 [1.36, 1.69] Complex measures of social integration 30 1.91 [1.63, 2.23] Combined structural and functional Multifaceted measurement 67 1.47 [1.34, 1.60] These analyses shifted the units of analysis, with distinct effect size estimates within studies used within different categories of measurement, such that many studies contributed more than one effect size but not more than one per category of measurement. OR, odds ratio, transformed from random effects weighted lnOR. Functional aspects of social relationships Twenty-four studies had data exclusive to functional measures of social relationships (see Figure 4). Across these studies, the random effects weighted average effect size was OR = 1.46 (95% CI = 1.28 to 1.66), which value fell within the CI of the omnibus results reported previously. There was moderate heterogeneity across studies (I2 = 47% [95% CI = 16% to 68%]; Q (23) = 44, p<0.01; τ2 = 0.04), so we conducted a random effects metaregression using the same variables and analytic procedures described previously. We extracted 87 unique effect sizes that were specific to measures of functional social relationships within 72 studies. A total of 16.5% of the variance in these effect sizes was explained in the metaregression, but the model did not reach statistical significance (p = 0.46). The results were not moderated by any of the specified participant characteristics (age, sex, initial health status, cause of mortality) or study characteristics (length of follow-up, geographic region, statistical controls). 10.1371/journal.pmed.1000316.g004 Figure 4 Forest plot of functional measures. Combined assessments of social relationships Sixty-one studies had combined data of both structural and functional measures of social relationships (see Figure 5). Across these studies, the random effects weighted average effect size was OR = 1.44 (95% CI = 1.32 to 1.58). A large degree of heterogeneity characterized studies (I2 = 82% [95% CI = 78% to 86%]; Q (60) = 337, p<0.001; τ2 = 0.09), and we conducted a random effects metaregression using the same variables and analytic procedures described previously. We extracted 64 unique effect sizes that evaluated combined structural and functional measures of social relationships within 61 studies. The metaregression explained only 6.8% of the variance in these effect sizes, and the model failed to reach statistical significance (p = 0.95). None of the variables in the metaregression moderated the results. 10.1371/journal.pmed.1000316.g005 Figure 5 Forest plot of combined measures. Discussion Cumulative empirical evidence across 148 independent studies indicates that individuals' experiences within social relationships significantly predict mortality. The overall effect size corresponds with a 50% increase in odds of survival as a function of social relationships. Multidimensional assessments of social integration yielded an even stronger association: a 91% increase in odds of survival. Thus, the magnitude of these findings may be considered quite large, rivaling that of well-established risk factors (Figure 6). Results also remained consistent across a number of factors, including age, sex, initial health status, follow-up period, and cause of death, suggesting that the association between social relationships and mortality may be generalized. 10.1371/journal.pmed.1000316.g006 Figure 6 Comparison of odds (lnOR) of decreased mortality across several conditions associated with mortality. Note: Effect size of zero indicates no effect. The effect sizes were estimated from meta analyses: ; A = Shavelle, Paculdo, Strauss, and Kush, 2008 [205]; B = Critchley and Capewell, 2003 [206]; C = Holman, English, Milne, and Winter, 1996 [207]; D = Fine, Smith, Carson, Meffe, Sankey, Weissfeld, Detsky, and Kapoor, 1994 [208]; E = Taylor, Brown, Ebrahim, Jollife, Noorani, Rees et al., 2004 [209]; F, G = Katzmarzyk, Janssen, and Ardern, 2003 [210]; H = Insua, Sacks, Lau, Lau, Reitman, Pagano, and Chalmers, 1994 [211]; I = Schwartz, 1994 [212]. The magnitude of risk reduction varied depending on the type of measurement of social relationships (see Table 4). Social relationships were most highly predictive of reduced risk of mortality in studies that included multidimensional assessments of social integration. Because these studies included more than one type of social relationship measurement (e.g., network based inventories, marital status, etc.), such a measurement approach may better represent the multiple pathways (described earlier) by which social relationships influence health and mortality [182]. Conversely, binary evaluations of living alone (yes/no) were the least predictive of mortality status. The reliability and validity of measurement likely explains this finding, and researchers are encouraged to use psychometrically sound measures of social relationships (e.g., Table 2). For instance, while researchers may be tempted to use a simple single-item such as “living alone” as a proxy for social isolation, it is possible for one to live alone but have a large supportive social network and thus not adequately capture social isolation. We also found that social isolation had a similar influence on likelihood of mortality compared with other measures of social relationships. This evidence qualifies the notion of a threshold effect (lack of social relationships is the only detrimental condition); rather, the association appears robust across a variety of types of measures of social relationships. This meta-analysis also provides evidence to support the directional influence of social relationships on mortality. Most of the studies (60%) involved community cohorts, most of whom would not be experiencing life-threatening conditions at the point of initial evaluation. Moreover, initial health status did not moderate the effect of social relationships on mortality. Although illness may result in poorer or more restricted social relationships (social isolation resulting from physical confinement), such that individuals closer to death may have decreased social support compared to healthy individuals, the findings from these studies indicate that general community samples with strong social relationships are likely to remain alive longer than similar individuals with poor social relations. However, causality is not easily established. One cannot randomly assign human participants to be socially isolated, married, or in a poor-quality relationship. A similar dilemma characterizes virtually all lifestyle risk factors for mortality: for instance, one cannot randomly assign individuals to be smokers or nonsmokers. Despite such challenges, “smoking represents the most extensively documented cause of disease ever investigated in the history of biomedical research” [183]. The link between social relationships and mortality is currently much less understood than other risk factors; nonetheless there is substantial experimental, cross-sectional, and prospective evidence linking social relationships with multiple pathways associated with mortality (see [182] for review). Existing models for reducing risk of mortality may be substantially strengthened by including social relationship factors. Notably, the overall effect for social relationships on mortality reported here may be a conservative estimate. Many studies included in the meta-analysis utilized single item measures of social relations, yet the magnitude of the association was greatest among those studies utilizing complex assessments. Moreover, because many studies statistically adjusted for standard risk factors, the effect may be underestimated, since some of the impact of social relationships on mortality may be mediated through such factors (e.g., behavior, diet, exercise). Additionally, most measures of social relations did not take into account the quality of the social relationships, thereby assuming that all relationships are positive. However, research suggests this is not the case, with negative social relationships linked to greater risk of mortality [184],[185]. For instance, marital status is widely used as a measure of social integration; however, a growing literature documents its divergent effects based on level of marital quality [186],[187]. Thus the effect of positive social relationships on risk of mortality may actually be much larger than reported in this meta-analysis, given the failure to account for negative or detrimental social relationships within the measures utilized across studies. Other possible limitations of this review should be acknowledged. Statistical controls (e.g., age, sex, physical condition, etc.) employed by many of the studies rule out a number of potentially confounding variables that might account for the association between social relationships and mortality. However, studies used an inconsistent variety of controlling variables, and some reports involved raw data (Table 1). Although effect size magnitude was diminished by the inclusion of statistical controls only within the data obtained by measures of structural social relationships (but not functional or combined measures), future research can better specify which variables are most likely to impact the overall association. It must also be acknowledged that existing data primarily represent research conducted in North America and Western Europe. Although we found no differences across world region, future reviews inclusive of research written in all languages (not only English) with participants better representing other world regions may yield better estimates across populations. Approximately two decades after the review by House and colleagues [1], a generation of empirical research validates their initial premise: Social relationships exert an independent influence on risk for mortality comparable with well established risk factors for mortality (Figure 6). Although limited by the state of current investigations and possible omission of pertinent reports, this meta-analysis provides empirical evidence (nearly 30 times the number of studies previously reported) to support the criteria for considering insufficient social relationships a risk factor of mortality (i.e., strength and consistency of association across a wide range of studies, temporal ordering, and gradient of response) [188]. The magnitude of the association between social relationships and mortality has now been established, and this meta-analysis provides much-needed clarification regarding the social relationship factor(s) most predictive of mortality. Future research can shift to more nuanced questions aimed at (a) understanding the causal pathways by which social participation promotes health, (b) refining conceptual models, and (c) developing effective intervention and prevention models that explicitly account for social relations. Some steps have already been taken identifying the psychological, behavioral, and physiological pathways linking social relationships to health [5],[182],[189]. Social relationships are linked to better health practices and to psychological processes, such as stress and depression, that influence health outcomes in their own right [190]; however, the influence of social relationships on health cannot be completely explained by these processes, as social relationships exert an independent effect. Reviews of such findings suggest that there are multiple biologic pathways involved (physiologic regulatory mechanisms, themselves intertwined) that in turn influence a number of disease endpoints [182],[191]–[193]. For instance, a number of studies indicate that social support is linked to better immune functioning [194]–[197] and to immune-mediated inflammatory processes [198]. Thus interdisciplinary work and perspective will be important in future studies given the complexity of the phenomenon. Perhaps the most important challenge posed by these findings is how to effectively utilize social relationships to reduce mortality risk. Preliminary investigations have demonstrated some risk reduction through formalized social interventions [199]. While the evidence is mixed [2],[6], it should be noted that most social support interventions evaluated in the literature thus far are based on support provided from strangers; in contrast, evidence provided in this meta-analysis is based almost entirely on naturally occurring social relationships. Moreover, our analyses suggest that received support is less predictive of mortality than social integration (Table 4). Therefore, facilitating patient use of naturally occurring social relations and community-based interventions may be more successful than providing social support through hired personnel, except in cases in which patient social relations appear to be detrimental or absent. Multifaceted community-based interventions may have a number of advantages because such interventions are socially grounded and include a broad cross-section of the public. Public policy initiatives need not be limited to those deemed “high risk” or those who have already developed a health condition but could potentially include low- and moderate-risk individuals earlier in the risk trajectory [200]. Overall, given the significant increase in rate of survival (not to mention quality of life factors), the results of this meta-analysis are sufficiently compelling to promote further research aimed at designing and evaluating interventions that explicitly account for social relationship factors across levels of health care (prevention, evaluation, treatment compliance, rehabilitation, etc.). Conclusion Data across 308,849 individuals, followed for an average of 7.5 years, indicate that individuals with adequate social relationships have a 50% greater likelihood of survival compared to those with poor or insufficient social relationships. The magnitude of this effect is comparable with quitting smoking and it exceeds many well-known risk factors for mortality (e.g., obesity, physical inactivity). These findings also reveal significant variability in the predictive utility of social relationship variables, with multidimensional assessments of social integration being optimal when assessing an individual's risk for mortality and evidence that social isolation has a similar influence on mortality to other measures of social relationships. The overall effect remained consistent across a number of factors, including age, sex, initial health status, follow-up period, and cause of death, suggesting that the association between social relationships and mortality may be general, and efforts to reduce risk should not be isolated to subgroups such as the elderly. To draw a parallel, many decades ago high mortality rates were observed among infants in custodial care (i.e., orphanages), even when controlling for pre-existing health conditions and medical treatment [201]–[204]. Lack of human contact predicted mortality. The medical profession was stunned to learn that infants would die without social interaction. This single finding, so simplistic in hindsight, was responsible for changes in practice and policy that markedly decreased mortality rates in custodial care settings. Contemporary medicine could similarly benefit from acknowledging the data: Social relationships influence the health outcomes of adults. Physicians, health professionals, educators, and the public media take risk factors such as smoking, diet, and exercise seriously; the data presented here make a compelling case for social relationship factors to be added to that list. With such recognition, medical evaluations and screenings could routinely include variables of social well-being; medical care could recommend if not outright promote enhanced social connections; hospitals and clinics could involve patient support networks in implementing and monitoring treatment regimens and compliance, etc. Health care policies and public health initiatives could likewise benefit from explicitly accounting for social factors in efforts aimed at reducing mortality risk. Individuals do not exist in isolation; social factors influence individuals' health though cognitive, affective, and behavioral pathways. Efforts to reduce mortality via social relationship factors will require innovation, yet innovation already characterizes many medical interventions that extend life at the expense of quality of life. Social relationship–based interventions represent a major opportunity to enhance not only the quality of life but also survival. Supporting Information Alternative Language Abstract S1 Abstract translated into Japanese by Hideko Cannell. (0.02 MB DOC) Click here for additional data file. Alternative Language Abstract S2 Abstract translated into Spanish by Rod Veas. (0.03 MB DOC) Click here for additional data file. Text S1 PRISMA checklist. (0.06 MB DOC) Click here for additional data file. Text S2 Review protocol. (0.05 MB DOC) Click here for additional data file.