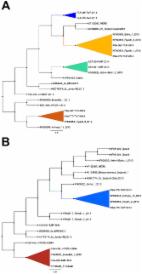
INTRODUCTION One hundred eighty laboratory-confirmed cases of human infection with Middle East respiratory syndrome coronavirus (MERS-CoV), 77 of them fatal, have been reported through 30 January 2014 (1) following the identification of the index case in the Kingdom of Saudi Arabia (KSA) in September 2012 (2). The majority of infections have been identified in the KSA with lower numbers in Jordan, Qatar, Tunisia, and the United Arab Emirates. Although cases have also been reported in France, Germany, Italy, and the United Kingdom, all have been linked to the Middle East either by travel of the individuals infected through an area where MERS-CoV has been reported or by direct or indirect contact with others who have a travel history consistent with exposure in the Middle East (3). Clusters of human infection indicate that human-to-human MERS-CoV transmission can occur (4, 5). However, the origin of the infection in most cases remains unknown. Analysis of human MERS-CoV sequences by Cotten et al. has revealed the presence of at least three circulating genotypes within the KSA alone (6). Phylogenetic analyses of 13 complete and 8 partial genome sequences enabled estimates of the timing and geographic origins of individual viral clades. The authors proposed that MERS-CoV emerged in humans in 2011 and noted that sequence divergence between clades is consistent with several sporadic introductions of the virus into the human population, presumably from an animal reservoir. Efforts to identify an animal reservoir have focused on bats and camels. Bats harbor a wide range of betacoronaviruses (7); furthermore, bat cell lines display the MERS-CoV receptor, dipeptidyl peptidase 4 (8), and can be experimentally infected. A short sequence fragment consistent with MERS-CoV was reported in a bat in Bisha, KSA, collected in close proximity to the home and workplace of the 2012 index case patient from whom the initial virus isolate was obtained (9). That same patient owned four pet dromedary camels (DC). Serological analysis of those DC revealed the presence of antibodies reactive with MERS-CoV; however, no MERS-CoV sequences were found by PCR analysis of nasal or rectal swabs or serum. Additional human cases have been associated with exposure to DC, and in some instances, investigators have described both serologic and genetic evidence of MERS-CoV infection in DC. Memish and coworkers reported PCR detection of MERS-CoV sequences in a DC with respiratory illness owned by an individual with MERS-CoV who had no history of contact with other infected humans (10). Haagmans et al. investigated an outbreak of the disease among humans on a Qatari farm and found MERS-CoV sequences in nasal swabs from 6 of 14 seropositive DC. Analysis of open reading frame 1a (ORF1a) and fragments representing ORF1b, spike, and ORF4b revealed similarity but not identity to sequences obtained from the MERS-CoV-infected humans at the same farm. The authors provide evidence that MERS-CoV can infect DC but cautiously conclude that data are insufficient to determine whether the infection spread from DC to humans, from humans to DC, or via another host to both species (11). Several groups have reported serological reactivity with MERS-CoV or a closely related virus in DC in the Middle East (12 – 15). Reusken et al. found antibodies in 100% of 50 Omani DC and 14% of 105 Canary Island DC but no seropositive northern European DC, domestic sheep, domestic goats, or domestic cattle (13). In two regions of the KSA, Hemida and colleagues detected antibodies to MERS-CoV in 90% of 310 DC but not in sheep, goats, cattle, or chickens. The seroprevalence was lower in DC 2 years of age. b Specimens collected from each animal: S, serum; B, blood; N, nasal swab; R, rectal swab. c Domestic sheep were separated into the breeds commonly found in the KSA: Barbari, Harri, Najdi, Naimi, and Sawakni. TABLE 2 Analysis of archived DC sera from the KSA from 1992 to 2010 Yr Location Age group No. % Seropositive (no. positive/total) 1992 Riyadh Adult 1 100 (1/1) 1993 Riyadh Adult 2 100 (2/2) 1994 Empty quarter Adult 123 93 (114/123) 1996 Riyadh Adult 6 100 (6/6) 2004 Riyadh Adult 6 100 (6/6) 2009 Riyadh Juvenile 56 72 (40/56) 2009 Rumah Adult 26 92 (24/26) 2010 Riyadh Juvenile 21 76 (16/21) 2010 Kharj Adult 23 91 (21/23) One hundred fifty (74%) of 203 DC sampled countrywide in 2013 were found to have antibodies to MERS-CoV by ELISA. The prevalence of seropositivity was higher in adult DC (>2 years of age; 93/98, 95%) than in juvenile DC (≤2 years of age; 57/104, 55%) (P 106 copies were all from juveniles, four of them from seronegative animals. The prevalence of PCR-positive DC ranged from 66% in Taif in the west to 0% in Gizan in the southwest. PCR analysis of a random selection of serum and whole blood samples collected from nasal or rectal swab PCR-positive, seropositive, and seronegative DC revealed no evidence of viremia (see Table S1 in the supplemental material). These included 13 adults and 29 juveniles phlebotomized in 2009, 15 adults and 14 juveniles phlebotomized in 2010, and 8 adults and 13 juveniles phlebotomized in 2013. These animals included the five juveniles with the highest viral genome sequence load in nasal swabs. Serum samples collected from goats (n = 36) and sheep (n = 112) in 2013 in the central region (Unizah, Riyadh) were not immunoreactive with MERS-CoV but were immunoreactive with Bo-CoV (25% of goats, n = 36; 54% of sheep, n = 24). Nasal swabs from 36 goats and 78 sheep were negative in RT-qPCR assays for MERS-CoV upE. To test the validity of RT-qPCR results and determine phylogenetic relationships of viral sequences found in DC in the KSA to previously reported sequences, we amplified and sequenced longer regions of the spike, ORF1ab, and nucleocapsid genes from RT-qPCR-positive samples (for the sequences of the primers used, see Table S2 in the supplemental material). Eleven of 13 swab samples with >105 copies in upE RT-qPCR yielded products for sequencing. No suitable products were obtained from samples with lighter viral sequence loads ( 80%, in juveniles, it ranged from 90% in the east to 5% in the southwest. The seroprevalence in DC ≤2 years of age was lower than that in older animals, confirming the results of Hemida et al. (15). Molecular analysis of nasal and rectal swab specimens indicated the highest prevalence of MERS-CoV sequences in DC in the west and northwest. Nasal swabs with heavy sequence loads (>105 copies) also clustered in the Taif region. A second sample collection in the west (Taif) separated from the first by an interval of 2 months confirmed the presence of heavy viral sequence loads in nasal swabs collected from juvenile animals sampled in this area (data not shown). These findings suggest that continuous, longer-term surveillance is necessary to determine the dynamics of virus circulation in DC populations. Lower prevalence rates of both MERS-CoV and Bo-CoV were evident in samples from the southwest. This may relate in part to the enforcement of restrictions of livestock movement in and out of Gizan Province implemented after the Rift Valley fever outbreak in 2000 but also to the generally lower DC population density in this region than in other regions of the KSA. Viral nucleic acids were more commonly detected in nasal swabs than in rectal specimens and were more frequent in juvenile than in adult animals. These findings, together with the absence of viremia and the known respiratory tract tropism of several other coronaviruses, suggest that airborne transmission is the most likely mode of MERS-CoV transmission. Although nucleic acid copy numbers were commonly highest in juvenile animals that were seronegative or had low antibody titers, positive findings were also obtained with specimens from highly seropositive and adult animals. Our findings in archived DC specimens, although restricted to serology, strongly suggest that MERS-CoV or a closely related virus has been circulating in DC in the KSA for at least 2 decades. Complete genomic sequences of MERS-CoV found in contemporary DC in the KSA are identical to sequences of viruses recovered from human MERS-CoV victims (unpublished data). Although we speculate that DC are potential reservoirs for human transmission, we cannot prove this relationship from the current data. Rigorous epidemiological investigation of the potential for exposure to DC in sporadic cases of MERS-CoV (those where there is no opportunity for human-to-human transmission) is required to test this model. If DC can be implicated, other questions will arise. Did MERS-CoV truly emerge as a human pathogen in 2012, or were cases of cryptic infection not appreciated because of a lack of suitable diagnostic tests? We may be able to address this conundrum by using archived human materials. If evidence of human MERS-CoV infections cannot be detected prior to 2012, we must entertain the possibility that mutation facilitated cross-species transmission. However, we see no path to address this possibility absent access to historical DC respiratory tract specimens. The only archived DC specimens we have been able to locate are DC sera; our efforts to recover MERS-CoV sequences from camel blood have been unsuccessful. What are the roles of bats, if any, as reservoirs of MERS-CoV? These limitations notwithstanding, the most urgent public health concern, raised in work we and others have reported that focuses on DC infection, is to determine the role of these animals in sporadic human infection. The evidence is clearly sufficient to support targeted investigation of direct or indirect exposure to DC in the disease among humans. MATERIALS AND METHODS Sample collection. Samples included DC, sheep, and goat sera; whole blood and nasal and rectal swabs freshly collected in 2013; and archived serum samples from 1992, 1993, 1994, 1996, 2004, 2009, and 2010. Two rectal and two nasal swabs were obtained from each animal. One rectal swab and one nasal swab were placed into RNAlater (Life Technologies, Carlsbad, CA), and one rectal swab and one nasal swab were placed into viral transport medium (Becton Dickinson, Franklin Lakes, NJ). All were stored at −80ºC. ELISA. Vero (African green monkey kidney, ATCC CRL-1586) cells were maintained at the Integrated Research Facility (Frederick, MD) in Dulbecco’s modified Eagle’s medium (Corning Inc., Corning, NY) and 10% fetal bovine serum. Cells were plated at a concentration of 4 × 104/well in 96-well plates (catalog no. 3603; Corning). When cells were at or near confluence, they were infected with the Jordan strain of MERS-CoV (GenBank accession no. KC776174, MERS-CoV Hu/Jordan-N3/2012[41]), kindly provided by Kanta Subbarao (National Institutes of Health, Bethesda, MD) and Gabriel Defang (Naval Medical Research Unit-3, Cairo, Egypt) at a multiplicity of infection of 1.0. At 24 h postinfection, cells were fixed in 10% neutral buffered formalin or 4% paraformaldehyde solution. After 24 h in fixative, plates were rinsed three times with phosphate-buffered saline (PBS) and placed in PBS for storage at 4°C. Plates were loaded with infected and noninfected cells in alternating rows to generate a differential reading for each serum tested. Positivity was defined as an infected-cell optical density of >0.6 and >3× the noninfected-cell optical density. Test sera were diluted 1:3,000 in PBS–0.05% Tween 20–1% bovine serum albumin; secondary antibodies were rabbit anti-goat IgG (H+L)-horseradish peroxidase conjugate (1:3,000; Bio-Rad, Hercules, CA), rabbit anti-sheep IgG (H+L)-horseradish peroxidase conjugate (1:3,000; Bio-Rad), and anti-llama IgG-horseradish peroxidase conjugate (1:10,000; Bethyl Laboratories, Montgomery, TX). Western blot. Extracts of noninfected Vero cells or Vero cells infected with MERS-CoV strain EMC were generated at Rocky Mountain Laboratories, loaded onto discontinuous 3 and 7.5% SDS gels (Bio-Rad), and transferred onto nitrocellulose with iBlot Transfer Stacks (Invitrogen iBlot; Life Technologies). Lanes were loaded with alternating infected and noninfected extract samples, and a pair were cut for incubation with DC sera (1:800 in blocking solution) after blocking of the membrane in PBS–0.05% Tween 20–5% dry milk blocking solution for 1 h. Membranes were washed three times with PBS–0.05% Tween 20 after a 2-h incubation with serum and then incubated for another 1.5 h with secondary antibody (1:7,000 in blocking solution; anti-llama IgG-horseradish peroxidase conjugate; Bethyl Laboratories). Following three more washes, the membranes were developed with WesternSure premium chemiluminescent substrate (LI-COR, Lincoln, NE) and read on a C-DiGit Blot Scanner (LI-COR). LIPS assay. The nucleocapsid proteins of Bo-CoV and MERS-CoV were PCR amplified with primers introducing appropriate restriction sites for cloning into vector pREN-2 fused to the C terminus of the Renilla luciferase reporter (20). Sequence-confirmed construct DNA was purified from Escherichia coli cultures (Qiagen, Hilden, Germany), and transfected into COS-1 cells (African green monkey kidney, ATCC CRL-1650; 1 µg; Lipofectamine; Invitrogen, Life Technologies). Cells were harvested at 48 h posttransfection in lysis buffer (50 mM Tris [pH 7.5], 100 mM NaCl, 5 mM MgCl2, 1% Triton X-100, 50% glycerol, protease inhibitors), and the protein extract was clarified by two rounds of centrifugation at 12,000 × g. The target concentration was determined in relative light units (RLU), and approximately 10 × 106 RLU were incubated with test serum (1:100) in a final volume of 100 µl buffer A (50 mM Tris [pH 7.7], 100 mM NaCl, 5 mM MgCl2, 1% Triton X-100) per well in 96-well plates (catalog no. 249944; Thermo/Nunc, Waltham, MA). After 1 h of incubation at room temperature, protein A/G beads (catalog no. 53135; Pierce, Junction City, OR) were added and the mixtures were transferred to 96-well filter plates (catalog no. MSBVN1B50; Millipore, Billerica, MA) for another hour of incubation at room temperature with shaking. Bead-bound antigen was washed eight times with buffer A and three times with PBS (Tecan Hydroflex, Maennedorf, Switzerland) and then read with coelenterazine substrate (Renilla luciferase assay system; Promega, Madison, WI) on a Centro LB960 luminometer (Berthold, Bad Wildbad, Germany). Nucleic acid extraction and PCR. Total nucleic acids were extracted from nasal swabs, serum, and whole blood on a QiaCube with Cador Reagent kits (Qiagen) or RNeasy Reagent kits for extraction of RNA from rectal swabs. Real-time qPCR used a OneStep Real-Time qPCR buffer (Invitrogen, Life Technologies) and primer/probes upE and ORF1a (16, 17). Products for sequencing were generated by RT-PCR. cDNA was reverse transcribed with Superscript III and random hexamer primers. PCR was performed with Amplitaq Gold (Life Technologies) and primers designed to amplify a 1,044-nt region of the spike gene (heminested PCR), a 913-nt region of the N gene (nested PCR) or a 2,004-nt region of the ORF1ab region (heminested PCR). For the sequences of the primers used, see Table S2 in the supplemental material. Products were purified by agarose gel electrophoresis and with QIAquick Gel Extraction kits (Qiagen) and subsequently sequenced on both strands by the dideoxynucleotide chain termination method (GeneWiz, South Plainfield, NJ). SUPPLEMENTAL MATERIAL Table S1 MERS-CoV infection in samples collected from Saudi Arabian animals in 2013. Table S1, DOCX file, 0.1 MB. Table S2 Sequences of primers used for PCR. Table S2, DOCX file, 0.1 MB.