- Record: found
- Abstract: found
- Article: not found
Vacancies in functional materials for clean energy storage and harvesting: the perfect imperfection
Read this article at
Abstract
Manipulating vacancies in functional materials offers scientists a powerful tool to design advanced materials for clean energy applications.
Abstract
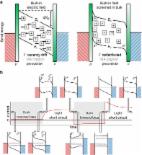
Vacancies exist throughout nature and determine the physical properties of materials. By manipulating the density and distribution of vacancies, it is possible to influence their physical properties such as band-gap, conductivity, magnetism, etc.This can generate exciting applications in the fields of water treatment, energy storage, and physical devices such as resistance-change memories. In this review, we focus on recent progress in vacancy engineering for the design of materials for energy harvesting applications. A brief discription of the concept of vacancies, the way to create and control them, as well as their fundamental properties, is first provided. Then, emphasis is placed on the strategies used to tailor vacancies for metal–insulator transitions, electronic structures, and introducing magnetism in non-magnetic materials. Finally, we present representative applications of different structures with vacancies as active electrode materials of lithium or sodium ion batteries, catalysts for water splitting, and hydrogen evolution.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells.
- Record: found
- Abstract: found
- Article: not found
Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies.
- Record: found
- Abstract: found
- Article: found
