- Record: found
- Abstract: found
- Article: found
A phase II study to evaluate the safety and efficacy of anlotinib combined with toripalimab for advanced biliary tract cancer
Read this article at
Abstract
Objectives
To assess the safety and efficacy of anlotinib (a multi‐targeted tyrosine kinase inhibitor) combined with toripalimab (a PD‐1 monoclonal antibody) in the treatment of unresectable biliary tract cancer (BTC).
Methods
In this prospective, single‐arm, single‐centre exploratory clinical study, patients with locally progressed or metastatic BTC were included. Patients were treated with anlotinib (12 mg, PO, QD, for 2 weeks and then stopped for a week, 21 days for a cycle) and toripalimab (240 mg, IV, Q3W). The primary endpoint of the study was the objective response rate (ORR), as defined in RECIST version 1.1 criteria.
Results
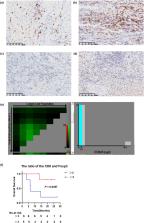
In this study, 15 BTC patients who met the criteria were enrolled. The ORR was 26.7%, the median progression‐free survival (mPFS) was 8.6 months (95% CI: 2.1–15.2), the median overall survival (mOS) was 14.53 months (95% CI: 0.8–28.2) and the disease control rate (DCR) was 87.6%. A patient with hilar cholangiocarcinoma was successfully converted after three cycles of treatment and underwent surgical resection. Furthermore, patient gene sequencing revealed that STK11 was mutated more frequently in patients with poor outcomes. In addition, patients with a CD8/Foxp3 ratio > 3 had a longer survival than those with a CD8/Foxp3 ratio ≤ 3 ( P = 0.0397).
Conclusions
In patients with advanced BTC, the combination of anlotinib and toripalimab demonstrated remarkable anti‐tumor potential, with increased objective response rates (ORR), longer overall survival (OS) and progression‐free survival (PFS). Moreover, STK11 and CD8/Foxp3 may be as biomarkers that can predict the effectiveness of targeted therapy in combination with immunotherapy.
Abstract
In this study, we found the combination of anlotinib (a multi‐targeted tyrosine kinase inhibitor) combined with toripalimab (a PD‐1 monoclonal antibody) demonstrated remarkable anti‐tumor potential in the treatment of unresectable biliary tract cancer (BTC). Moreover, STK11 and CD8/Foxp3 may be as biomarkers that can predict the effectiveness of targeted therapy in combination with immunotherapy.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study
- Record: found
- Abstract: found
- Article: not found
Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy.
- Record: found
- Abstract: not found
- Article: not found
