- Record: found
- Abstract: found
- Article: found
Farnesyl diphosphate synthase is important for the maintenance of glioblastoma stemness
Read this article at
Abstract
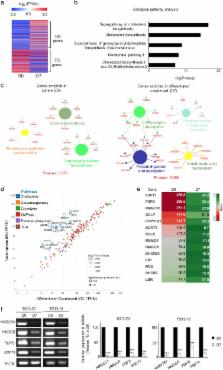
Glioblastoma is a highly malignant tumor that easily acquires resistance to treatment. The stem-cell-like character (stemness) has been thought to be closely associated with the treatment resistance of glioblastoma cells. In this study, we determined that farnesyl diphosphate synthase (FDPS), a key enzyme in isoprenoid biosynthesis, plays an important role in maintaining glioblastoma stemness. A comparison of the mRNA expression in patient-derived glioblastoma sphere cells, which maintain stemness, and their differentiated counterparts, which lose stemness, via RNA sequencing showed that most of the altered genes were networked in the cholesterol biosynthesis pathway. We screened Federal Drug Administration (FDA)-approved drugs targeting specific enzymes in the cholesterol biosynthesis pathway for their ability to inhibit glioblastoma sphere formation. Inhibitors of FDPS, such as alendronate and zoledronate, significantly reduced the formation of glioblastoma spheres, and alendronate was effective at a lower molar concentration than zoledronate. Knockdown of FDPS using short hairpin RNA also completely inhibited the formation of secondary spheres. FDPS mRNA in patients with glioblastoma was associated with malignancy in three independent microarray data sets. RNA sequencing showed that alendronate treatment reduced the embryonic stem cell signature and activated development- and necrosis-related pathways in glioblastoma spheres. These results suggest that FDPS is important for the maintenance of glioblastoma stemness and that alendronate, a drug widely used to treat osteoporosis, can be repositioned to treat glioblastoma.
Brain cancer: Enzyme target for potential therapy
A drug that targets a key enzyme in aggressive brain cancer tumors could help tackle resistance to existing treatments. Glioblastoma is the most aggressive form of brain cancer and remains difficult to treat because the cancer cells can survive chemotherapy and radiotherapy. Certain cells within glioblastoma tumors have ‘stemness’ – unique stem cell-like metabolic characteristics that allow them to rapidly repair DNA damage and trigger relapse. Hyonchol Jang at the National Cancer Center in Goyang, South Korea and co-workers discovered that an enzyme called farnesyl diphosphate synthase (FDPS) helps maintain stemness in glioblastoma. The team then treated patient-derived glioblastoma cells with existing drugs known to inhibit FDPS. One such drug, which is already used to treat osteoporosis, inhibited the formation of secondary glioblastoma and may prove valuble in the treatment of brain cancer.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Evolution of the cancer stem cell model.
- Record: found
- Abstract: found
- Article: not found
Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation
- Record: found
- Abstract: found
- Article: not found