- Record: found
- Abstract: found
- Article: found
Evaluating Quality Management and Diagnostics Microbiology Performance Within an International External Quality Assessment (EQA) Program Serving National One Health Sector Reference Laboratories Across Asia: Experience Amid the Coronavirus Disease 2019 (COVID-19) Pandemic
Read this article at
Abstract
Background
Strengthening external quality assessment (EQA) services across the One Health sector supports implementation of effective antimicrobial resistance (AMR) control strategies. Here we describe and compare 2 different approaches for conducting virtual laboratory follow-up assessments within an EQA program to evaluate quality management system (QMS) and procedures for pathogen identification and antimicrobial susceptibility testing (AST).
Methods
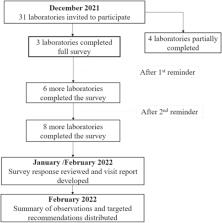
During the coronavirus disease 2019 (COVID-19) pandemic in 2021 and 2022, 2 laboratory assessment approaches were introduced: virtual-based and survey-based methodologies. The evaluation of 2 underperforming Animal Health laboratories through a virtual-based approach occurred between May and August 2021. This evaluation encompassed the utilization of 3 online meetings and document reviews, performed subsequent to the execution of EQA procedures. Within a distinct group of laboratories, the survey-based assessment was implemented from December 2021 to February 2022, also following EQA procedures. This phase encompassed the dissemination of an online survey to 31 participating laboratories, alongside a sole online consultation meeting involving 4 specific underperforming laboratories.
Results
The virtual-based assessment post-EQA aimed to identify gaps and areas for improvement in the laboratory's practices for pathogen identification and AST. This approach was, however, time-intensive, and, hence, only 2 laboratories were assessed. In addition, limited interactions in virtual platforms compromised the assessment quality. The survey-based post-EQA assessment enabled evaluation of 31 laboratories. Despite limitations for in-depth analysis of each procedure, gaps in QMS across multiple laboratories were identified and tailored laboratory-specific recommendations were provided.
Conclusions
Reliable internet and plans for efficient time management, post-EQA virtual laboratory follow-up assessments are an effective alternative when conducting onsite evaluation is infeasible as observed during the COVID-19 pandemic, although the successful implementation of remediation plans will likely require in person assessments. We advocate application of hybrid approaches (both onsite and virtual) for targeted capacity building of AMR procedures with the ability to implement and oversee the process.
Abstract
External quality assessment (EQA) is crucial for quality assurance in antimicrobial resistance (AMR) surveillance and effective control interventions. Optimized capacity-building approaches are needed post-EQA for different scenarios, including pandemics with travel restrictions.
Related collections
Most cited references12
- Record: found
- Abstract: found
- Article: found
External quality assessment of national public health laboratories in Africa, 2002-2009
- Record: found
- Abstract: found
- Article: not found
Implementation of quality management for clinical bacteriology in low-resource settings.
- Record: found
- Abstract: not found
- Book: not found