- Record: found
- Abstract: found
- Article: found
Demographic and Health Behavior Factors Associated With Clinical Trial Invitation and Participation in the United States
Read this article at
Key Points
Question
What person-level factors are associated with US adults’ invitation to and participation in clinical trials?
Findings
In this cross-sectional study of 3689 adults, 9% were invited to participate in a clinical trial, and of those, 47% participated. Respondents had higher odds of clinical trial invitation if they were non-Hispanic Black, college educated, single, or urban-dwelling or had medical conditions; non-Hispanic Black respondents had lower odds of clinical trial participation.
Abstract
This cross-sectional study examines demographic, clinical, and health behavior–related factors associated with invitation to and participation in clinical trials in the US.
Abstract
Importance
Representative enrollment in clinical trials is critical to ensure equitable and effective translation of research to practice, yet disparities in clinical trial enrollment persist.
Objective
To examine person-level factors associated with invitation to and participation in clinical trials.
Design, Setting, and Participants
This cross-sectional study analyzed responses from 3689 US adults who participated in the nationally representative Health Information National Trends Survey, collected February through June 2020 via mailed questionnaires.
Main Outcomes and Measures
History of invitation to and participation in a clinical trial, primary information sources, trust in information sources, and motives for participation in clinical trials were described. Respondent characteristics are presented as absolute numbers and weighted percentages. Associations between respondent demographic, clinical, and health behavior–related characteristics and clinical trial invitation and participation were estimated using survey-weighted logistic regression models.
Results
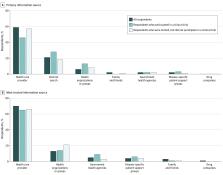
The median (IQR) age of the 3689 respondents was 48 (33-61) years, and most were non-Hispanic White individuals (2063 [59%]; non-Hispanic Black, 452 [10%]; Hispanic, 521 [14%]), had more than a high school degree (2656 [68%]), were employed (1809 [58%]), and had at least 1 medical condition (2535 [61%]). Overall, 439 respondents (9%) had been invited to participate in any clinical trial. Respondents with increased odds of invitation were non-Hispanic Black compared with non-Hispanic White (adjusted odds ratio [aOR], 1.85; 95% CI, 1.13-3.02), had greater than a high school education compared with less than high school education (eg, ≥college degree: aOR, 4.84; 95% CI, 1.89-12.39), were single compared with married or living as married (aOR, 1.68; 95% CI, 1.04-2.73), and had at least 1 medical condition compared to none (eg, 1 medical condition: aOR, 2.25; 95% CI, 1.32-3.82). Respondents residing in rural vs urban areas had 77% decreased odds of invitation to a clinical trial (aOR 0.33; 95% CI 0.17-0.65). Of invited respondents, 199 (47%) participated. Compared with non-Hispanic White respondents, non-Hispanic Black respondents had 72% decreased odds of clinical trial participation (aOR, 0.28; 95% CI, 0.09-0.87). Respondents most frequently reported “health care providers” as the first and most trusted source of clinical trial information (first source: 2297 [59%]; most trusted source: 2597 [70%]). The most frequently reported motives for clinical trials participation were “wanting to get better” (2294 [66%]) and the standard of care not being covered by insurance (1448 [41%]).
Conclusions and Relevance
The findings of this study suggest that invitation to and participation in clinical trials may differ by person-level demographic and clinical characteristics. Strategies toward increasing trial invitation and participation rates across diverse patient populations warrant further research to ensure equitable translation of clinical benefits from research to practice.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies.
- Record: found
- Abstract: found
- Article: found