- Record: found
- Abstract: found
- Article: not found
Diagnostic Testing for Severe Acute Respiratory Syndrome–Related Coronavirus-2 : A Narrative Review
Abstract
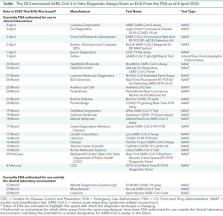
Diagnostic testing to identify persons infected with severe acute respiratory syndrome–related coronavirus-2 (SARS–CoV-2) infection is central to control the global pandemic of COVID-19 that began in late 2019. In a few countries, the use of diagnostic testing on a massive scale has been a cornerstone of successful containment strategies. In contrast, the United States, hampered by limited testing capacity, has prioritized testing for specific groups of persons. Real-time reverse transcriptase polymerase chain reaction–based assays performed in a laboratory on respiratory specimens are the reference standard for COVID-19 diagnostics. However, point-of-care technologies and serologic immunoassays are rapidly emerging. Although excellent tools exist for the diagnosis of symptomatic patients in well-equipped laboratories, important gaps remain in screening asymptomatic persons in the incubation phase, as well as in the accurate determination of live viral shedding during convalescence to inform decisions to end isolation. Many affluent countries have encountered challenges in test delivery and specimen collection that have inhibited rapid increases in testing capacity. These challenges may be even greater in low-resource settings. Urgent clinical and public health needs currently drive an unprecedented global effort to increase testing capacity for SARS–CoV-2 infection. Here, the authors review the current array of tests for SARS–CoV-2, highlight gaps in current diagnostic capacity, and propose potential solutions.
Abstract
Diagnostic testing to identify infected persons is central to efforts to control the COVID-19 pandemic. This review synthesizes current knowledge of diagnostic options available to clinicians, highlights key gaps in current diagnostic capacity, and discusses potential solutions.
Related collections
Most cited references8
- Record: found
- Abstract: found
- Article: not found
Diagnostic Accuracy of Rapid Antigen Detection Tests for Respiratory Syncytial Virus Infection: Systematic Review and Meta-analysis.

- Record: found
- Abstract: found
- Article: found
A sensitive and specific antigen detection assay for Middle East respiratory syndrome coronavirus
- Record: found
- Abstract: found
- Article: found
