- Record: found
- Abstract: found
- Article: found
An Autoimmune Disease-Associated Risk Variant in the TNFAIP3 Gene Plays a Protective Role in Brucellosis That Is Mediated by the NF-κB Signaling Pathway
Read this article at
ABSTRACT
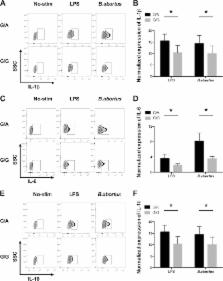
Naturally occurring functional variants (rs148314165 and rs200820567, collectively referred to as TT>A) reduce the expression of the tumor necrosis factor alpha-induced protein 3 ( TNFAIP3) gene, a negative regulator of NF-κB signaling, and predispose individuals to autoimmune disease. In this analysis, we conducted a genetic association study of the TT>A variants in 1,209 controls and 150 patients with brucellosis, an infectious disease, and further assessed the role of the variants in brucellosis. Our data demonstrated that the TT>A variants were correlated with cases of brucellosis ( P = 0.002; odds ratio [OR] = 0.34) and with individuals who had a positive serum agglutination test (SAT) result (titer of >1/160) ( P = 4.2 × 10 −6; OR = 0.23). A functional study demonstrated that brucellosis patients carrying the protective allele (A) showed significantly lower expression levels of the TNFAIP3 gene in their peripheral blood mononuclear cells and showed increased NF-κB signaling. Monocytes from individuals carrying the A allele that were stimulated with Brucella abortus had lower mRNA levels of TNFAIP3 and produced more interleukin-10 (IL-10), IL-6, and IL-1β than those from TT allele carriers. These data showed that autoimmune disease-associated risk variants, TT>A, of the TNFAIP3 locus play a protective role in the pathogenesis of brucellosis. Our findings suggest that a disruption of the normal function of the TNFAIP3 gene might serve as a therapeutic target for the treatment of brucellosis.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Treating inflammation by blocking interleukin-1 in humans.
- Record: found
- Abstract: found
- Article: not found
Brucella abortus Uses a Stealthy Strategy to Avoid Activation of the Innate Immune System during the Onset of Infection
- Record: found
- Abstract: found
- Article: not found