- Record: found
- Abstract: found
- Article: found
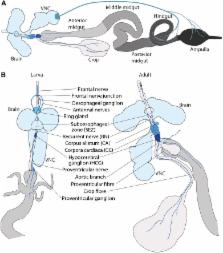
Anatomy and Physiology of the Digestive Tract of Drosophila melanogaster
Read this article at
Abstract
The gastrointestinal tract has recently come to the forefront of multiple research fields. It is now recognized as a major source of signals modulating food intake, insulin secretion and energy balance. It is also a key player in immunity and, through its interaction with microbiota, can shape our physiology and behavior in complex and sometimes unexpected ways. The insect intestine had remained, by comparison, relatively unexplored until the identification of adult somatic stem cells in the Drosophila intestine over a decade ago. Since then, a growing scientific community has exploited the genetic amenability of this insect organ in powerful and creative ways. By doing so, we have shed light on a broad range of biological questions revolving around stem cells and their niches, interorgan signaling and immunity. Despite their relatively recent discovery, some of the mechanisms active in the intestine of flies have already been shown to be more widely applicable to other gastrointestinal systems, and may therefore become relevant in the context of human pathologies such as gastrointestinal cancers, aging, or obesity. This review summarizes our current knowledge of both the formation and function of the Drosophila melanogaster digestive tract, with a major focus on its main digestive/absorptive portion: the strikingly adaptable adult midgut.
Related collections
Most cited references388
- Record: found
- Abstract: found
- Article: not found
Using FlyAtlas to identify better Drosophila melanogaster models of human disease.
- Record: found
- Abstract: found
- Article: not found
Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut.
- Record: found
- Abstract: found
- Article: not found