- Record: found
- Abstract: found
- Article: found
PINK1 autophosphorylation upon membrane potential dissipation is essential for Parkin recruitment to damaged mitochondria

Read this article at
Abstract
Dysfunction of PINK1, a mitochondrial Ser/Thr kinase, causes familial Parkinson's disease (PD). Recent studies have revealed that PINK1 is rapidly degraded in healthy mitochondria but accumulates on the membrane potential (ΔΨm)-deficient mitochondria, where it recruits another familial PD gene product, Parkin, to ubiquitylate the damaged mitochondria. Despite extensive study, the mechanism underlying the homeostatic control of PINK1 remains unknown. Here we report that PINK1 is autophosphorylated following a decrease in ΔΨm and that most disease-relevant mutations hinder this event. Mass spectrometric and mutational analyses demonstrate that PINK1 autophosphorylation occurs at Ser228 and Ser402, residues that are structurally clustered together. Importantly, Ala mutation of these sites abolishes autophosphorylation of PINK1 and inhibits Parkin recruitment onto depolarized mitochondria, whereas Asp (phosphorylation-mimic) mutation promotes mitochondrial localization of Parkin even though autophosphorylation was still compromised. We propose that autophosphorylation of Ser228 and Ser402 in PINK1 is essential for efficient mitochondrial localization of Parkin.
Abstract
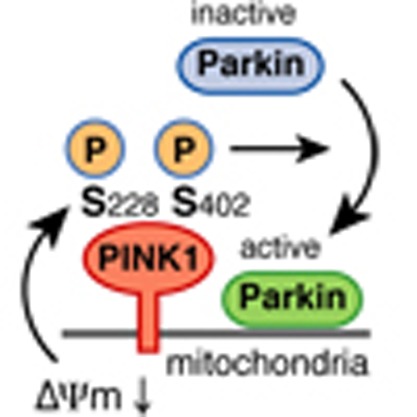 The kinase PINK1 is mutated in Parkinson's disease and accumulates in defective mitochondria,
where it recruits Parkin. Here, PINK1 is shown to be autophosphorylated and this is
required for the localization of PINK1 to mitochondria with a reduced membrane potential,
and for the recruitment of Parkin.
The kinase PINK1 is mutated in Parkinson's disease and accumulates in defective mitochondria,
where it recruits Parkin. Here, PINK1 is shown to be autophosphorylated and this is
required for the localization of PINK1 to mitochondria with a reduced membrane potential,
and for the recruitment of Parkin.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Phosphate-binding tag, a new tool to visualize phosphorylated proteins.
- Record: found
- Abstract: found
- Article: not found
The conformational plasticity of protein kinases.
- Record: found
- Abstract: found
- Article: not found