- Record: found
- Abstract: found
- Article: found
High adherence to the ‘Wise List’ treatment recommendations in Stockholm: a 15-year retrospective review of a multifaceted approach promoting rational use of medicines
Read this article at
Abstract
Objectives
To present the ‘Wise List’ (a formulary of essential medicines for primary and specialised care in Stockholm Healthcare Region) and assess adherence to the recommendations over a 15-year period.
Design
Retrospective analysis of all prescription data in the Stockholm Healthcare Region between 2000 and 2015 in relation to the Wise List recommendations during the same time period.
Main outcome measures
The number of core and complementary substances included in the Wise List, the adherence to recommendations by Anatomic Therapeutic Chemical (ATC) 1st level using defined daily doses (DDDs) adjusted to the DDD for 2015, adherence to recommendations over time measured by dispensed prescriptions yearly between 2002 and 2015.
Results
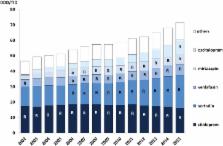
The number of recommended core substances was stable (175–212). Overall adherence to the recommendations for core medicines for all prescribers increased from 75% to 84% (2000 to 2015). The adherence to recommendations in primary care for core medicines increased from 80% to 90% (2005 to 2015) with decreasing range in practice variation (32% to 13%). Hospital prescriber adherence to core medicine recommendations was stable but increased for the combination core and complementary medicines from 77% to 88% (2007 to 2015). Adherence varied between the 4 therapeutic areas studied.
Conclusions
High and increasing adherence to the Wise List recommendations was seen for all prescriber categories. The transparent process for developing recommendations involving respected experts and clinicians using strict criteria for handling potential conflicts of interests, feedback to prescribers, continuous medical education and financial incentives are possible contributing factors. High-quality evidence-based recommendations to prescribers, such as the Wise List, disseminated through a multifaceted approach, will become increasingly important and should be developed further to include recommendations and introduction protocols for new expensive medicines.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months.
- Record: found
- Abstract: found
- Article: not found
From best evidence to best practice: effective implementation of change in patients' care
- Record: found
- Abstract: found
- Article: not found