- Record: found
- Abstract: found
- Article: found
Incidence and prevalence of vaccine preventable infections in adult patients with autoimmune inflammatory rheumatic diseases (AIIRD): a systemic literature review informing the 2019 update of the EULAR recommendations for vaccination in adult patients with AIIRD
Read this article at
Abstract
Objectives
The aims of this study were to update the evidence on the incidence and prevalence rates of vaccine preventable infections (VPI) in patients with autoimmune inflammatory rheumatic diseases (AIIRD) and compare the data to the general population when available.
Methods
A literature search was performed using Medline, Embase and Cochrane library (October 2009 to August 2018). The primary outcome was the incidence or prevalence of VPI in the adult AIIRD population. Meta-analysis was performed when appropriate.
Results
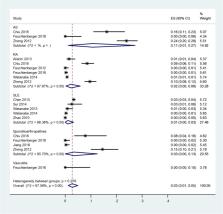
Sixty-three publications out of 3876 identified records met the inclusion criteria: influenza (n=4), pneumococcal disease (n=7), hepatitis B (n=10), herpes zoster (HZ) (n=29), human papillomavirus (HPV) infection (n=13). An increased incidence of influenza and pneumococcal disease was reported in patients with AIIRD. HZ infection-pooled incidence rate ratio (IRR) was 2.9 (95% CI 2.4 to 3.3) in patients with AIIRD versus general population. Among AIIRD, inflammatory myositis conferred the highest incidence rate (IR) of HZ (pooled IRR 5.1, 95% CI 4.3 to 5.9), followed by systemic lupus erythematosus (SLE) (pooled IRR 4.0, 95% CI 2.3 to 5.7) and rheumatoid arthritis (pooled IRR 2.3, 95% CI 2.1 to 2.6). HPV infection-pooled prevalence ratio was 1.6, 95% CI 0.7 to 3.4 versus general population, based on studies mainly conducted in the SLE population in Latin America and Asia. Pooled prevalence of hepatitis B surface antigen and hepatitis B core antibody in patients with AIIRD was similar to the general population, 3%, 95% CI 1% to 5% and 15%, 95% CI 7% to 26%, respectively.
Related collections
Most cited references78

- Record: found
- Abstract: found
- Article: found
Anifrolumab, an Anti–Interferon‐α Receptor Monoclonal Antibody, in Moderate‐to‐Severe Systemic Lupus Erythematosus
- Record: found
- Abstract: found
- Article: not found
Incidence and prevalence of hepatitis C in prisons and other closed settings: results of a systematic review and meta-analysis.
- Record: found
- Abstract: found
- Article: not found