- Record: found
- Abstract: found
- Article: found
Racial and ethnic disparities in COVID-19 booster vaccination among U.S. older adults differ by geographic region and Medicare enrollment
Read this article at
Abstract
Introduction
COVID-19 booster vaccines are highly effective at reducing severe illness and death from COVID-19. Research is needed to identify whether racial and ethnic disparities observed for the primary series of the COVID-19 vaccines persist for booster vaccinations and how those disparities may vary by other characteristics. We aimed to measure racial and ethnic differences in booster vaccine receipt among U.S. Medicare beneficiaries and characterize potential variation by demographic characteristics.
Methods
We conducted a cohort study using CVS Health and Walgreens pharmacy data linked to Medicare claims. We included community-dwelling Medicare beneficiaries aged ≥66 years who received two mRNA vaccine doses (BNT162b2 and mRNA-1273) as of 8/1/2021. We followed beneficiaries from 8/1/2021 until booster vaccine receipt, death, Medicare disenrollment, or end of follow-up (12/31/2021). Adjusted Poisson regression was used to estimate rate ratios (RRs) and 95% confidence intervals (CIs) comparing vaccine uptake between groups.
Results
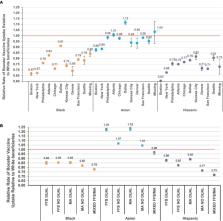
We identified 11,339,103 eligible beneficiaries (mean age 76 years, 60% female, 78% White). Overall, 67% received a booster vaccine (White = 68.5%; Asian = 67.0%; Black = 57.0%; Hispanic = 53.3%). Compared to White individuals, Black (RR = 0.78 [95%CI = 0.78–0.78]) and Hispanic individuals (RR = 0.72 [95% = CI 0.72–0.72]) had lower rates of booster vaccination. Disparities varied by geographic region, urbanicity, and Medicare plan/Medicaid eligibility. The relative magnitude of disparities was lesser in areas where vaccine uptake was lower in White individuals.
Related collections
Most cited references27

- Record: found
- Abstract: found
- Article: found
Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England
- Record: found
- Abstract: found
- Article: not found
BNT162b2 Vaccine Booster and Mortality Due to Covid-19
- Record: found
- Abstract: found
- Article: not found