- Record: found
- Abstract: found
- Article: found
microRNA Crosstalk Influences Epithelial-to-Mesenchymal, Endothelial-to-Mesenchymal, and Macrophage-to-Mesenchymal Transitions in the Kidney
Read this article at
Abstract
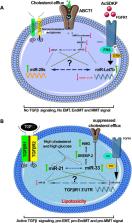
microRNAs (miRNAs) are small, non-coding nucleotides that regulate diverse biological processes. Altered microRNA biosynthesis or regulation contributes to pathological processes including kidney fibrosis. Kidney fibrosis is characterized by deposition of excess extracellular matrix (ECM), which is caused by infiltration of immune cells, inflammatory cells, altered chemokines, and cytokines as well as activation and accumulation of fibroblasts in the kidney. These activated fibroblasts can arise from epithelial cells via epithelial-to-mesenchymal transition (EMT), from bone marrow-derived M2 phenotype macrophages via macrophage-to-mesenchymal transition (MMT), from endothelial cells via endothelial-to-mesenchymal transition (EndMT), from resident fibroblasts, and from bone marrow-derived monocytes and play a crucial role in fibrotic events. Disrupted microRNA biosynthesis and aberrant regulation contribute to the activation of mesenchymal programs in the kidney. miR-29 regulates the interaction between dipeptidyl peptidase-4 (DPP-4) and integrin β1 and the associated active transforming growth factor β (TGFβ) and pro-EndMT signaling; however, miR-let-7 targets transforming growth factor β receptors (TGFβRs) to inhibit TGFβ signaling. N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP) is an endogenous anti-fibrotic peptide, which is associated with fibroblast growth factor receptor 1 (FGFR1) phosphorylation and subsequently responsible for the production of miR-let-7. miR-29 and miR-let-7 family clusters participate in crosstalk mechanisms, which are crucial for endothelial cell homeostasis. The physiological level of AcSDKP is vital for the activation of anti-fibrotic mechanisms including restoration of anti-fibrotic microRNA crosstalk and suppression of profibrotic signaling by mitigating DPP-4-associated mesenchymal activation in the epithelial cells, endothelial cells, and M2 phenotype macrophages. The present review highlights recent advancements in the understanding of both the role of microRNAs in the development of kidney disease and their potential as novel therapeutic targets for fibrotic disease states.
Related collections
Most cited references141
- Record: found
- Abstract: found
- Article: not found
Regulation of microRNA function in animals
- Record: found
- Abstract: found
- Article: not found
Origin and function of myofibroblasts in kidney fibrosis.
- Record: found
- Abstract: found
- Article: not found