- Record: found
- Abstract: found
- Article: found
Identification of an Immature Subset of PMN-MDSC Correlated to Response to Checkpoint Inhibitor Therapy in Patients with Metastatic Melanoma
Read this article at
Abstract
Simple Summary
Polymorphonuclear myeloid-derived suppressive cells (PMN-MDSCs) have been associated to bad prognosis and resistance to immune checkpoint inhibitor (ICI) therapy in metastatic melanoma (MM). In this study, we describe an immature subset of PMN-MDSCs capable of a high cytotoxicity against T cells mediated by a MAC-1 interaction, characterized by the absence of expression of the signal regulatory protein alpha. This subset is increased in patients responding to ICI therapy. Although the processes involving these cells in vivo are unknown, low-density CD15 +SIRPα − cells might constitute a useful biomarker to monitor clinical response in MM patients.
Abstract
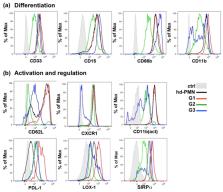
PMN-MDSCs support tumor progression and resistance to ICI therapy through their suppressive functions but their heterogeneity limits their use as biomarkers in cancer. Our aim was to investigate the phenotypic and functional subsets of PMN-MDSCs to identify biomarkers of response to ICI therapy. We isolated low-density CD15 + PMNs from patients with metastatic melanoma and assessed their immune-suppressive capacities. Expression of CD10 and CD16 was used to identify mature and immature subsets and correlate them to inhibition of T cell proliferation or direct cytotoxicity. Frequencies of the PMN-MDSCs subsets were next correlated to the radiological response of 36 patients receiving ICI therapy. Mature activated cells constituted the major population of PMN-MDSCs. They were found in a higher proportion in the pre-treatment blood of patients non responders to ICI. A subset of immature cells characterized by intermediate levels of CD10 and CD16, the absence of expression of SIRPα and a strong direct cytotoxicity to T cells was increased in patients responding to ICI. The paradoxical expansion of such cells during ICI therapy suggests a role of PMNs in the inflammatory events associated to efficient ICI therapy and the usefulness of their monitoring in patients care.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
The blockade of immune checkpoints in cancer immunotherapy.

- Record: found
- Abstract: found
- Article: found