- Record: found
- Abstract: found
- Article: found
FGF18 alleviates hepatic ischemia-reperfusion injury via the USP16-mediated KEAP1/Nrf2 signaling pathway in male mice
Read this article at
Abstract
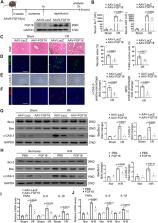
Hepatic ischemia-reperfusion injury (IRI) is a common complication occurs during hepatic resection and transplantation. However, the mechanisms underlying hepatic IRI have not been fully elucidated. Here, we aim to explore the role of fibroblast growth factor 18 (FGF18) in hepatic IRI. In this work, we find that Hepatic stellate cells (HSCs) secrete FGF18 and alleviates hepatocytes injury. HSCs-specific FGF18 deletion largely aggravates hepatic IRI. Mechanistically, FGF18 treatment reduces the levels of ubiquitin carboxyl-terminal hydrolase 16 (USP16), leading to increased ubiquitination levels of Kelch Like ECH Associated Protein 1 (KEAP1) and the activation of nuclear factor erythroid 2-related factor 2 (Nrf2). Furthermore, USP16 interacts and deubiquitinates KEAP1. More importantly, Nrf2 directly binds to the promoter of USP16 and forms a negative feedback loop with USP16. Collectively, our results show FGF18 alleviates hepatic IRI by USP16/KEAP1/Nrf2 signaling pathway in male mice, suggesting that FGF18 represents a promising therapeutic approach for hepatic IRI.
Abstract
Hepatic ischemia-reperfusion injury (IRI) is a common complication that occurs during hepatic resection and transplantation. Here the authors show that Hepatic stellate cells secrete FGF18 which alleviates hepatocyte injury by USP16/KEAP1/Nrf2 signaling.
Related collections
Most cited references44

- Record: found
- Abstract: found
- Article: found
Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription
- Record: found
- Abstract: found
- Article: not found
High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers.
- Record: found
- Abstract: found
- Article: not found