- Record: found
- Abstract: found
- Article: found
Liposomal Formulations in Clinical Use: An Updated Review

Read this article at
Abstract
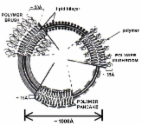
Liposomes are the first nano drug delivery systems that have been successfully translated into real-time clinical applications. These closed bilayer phospholipid vesicles have witnessed many technical advances in recent years since their first development in 1965. Delivery of therapeutics by liposomes alters their biodistribution profile, which further enhances the therapeutic index of various drugs. Extensive research is being carried out using these nano drug delivery systems in diverse areas including the delivery of anti-cancer, anti-fungal, anti-inflammatory drugs and therapeutic genes. The significant contribution of liposomes as drug delivery systems in the healthcare sector is known by many clinical products, e.g., Doxil ®, Ambisome ®, DepoDur™, etc. This review provides a detailed update on liposomal technologies e.g., DepoFoam™ Technology, Stealth technology, etc., the formulation aspects of clinically used products and ongoing clinical trials on liposomes.
Related collections
Most cited references145
- Record: found
- Abstract: found
- Article: not found
Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications.
- Record: found
- Abstract: found
- Article: not found
To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine?
- Record: found
- Abstract: found
- Article: found