- Record: found
- Abstract: found
- Article: found
Cancer Drugs Reimbursed with Limited Evidence on Overall Survival and Quality of Life: Do Follow-Up Studies Confirm Patient Benefits?
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Background and Objective
Cancer drug costs have increased considerably within healthcare systems, but many
drugs lack quality-of-life (QoL) and overall survival (OS) data at the time of reimbursement
approval. This study aimed to review the extent of subsequent literature documenting
improvements in OS and QoL for cancer drug indications where no such evidence existed
at the time of reimbursement approval.
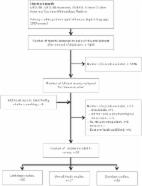
Methods
Drug indications with claims of added therapeutical value but a lack of evidence on
OS and QoL that were reimbursed between 2010 and 2020 in Sweden were included for
review. Searches were conducted in PubMed and ClinicalTrial.gov for randomized controlled
trials examining OS and QoL.
Results
Of the 22 included drug indications, seven were found to have at least one trial with
conclusive evidence of improvements in OS or QoL after a mean follow-up of 6.6 years.
The remaining 15 drug indications either lacked subsequent randomized controlled trial
data on OS or QoL (n = 6) or showed no statistically significant improvements (n =
9). Only one drug demonstrated evidence of improvement in both OS and QoL for its
indication.
Conclusions
A considerable share of reimbursed cancer drug indications continue to lack evidence
of improvement in both OS and QoL. With limited healthcare resources and an increasing
cancer burden, third-party payers have strong incentives to require additional post-reimbursement
data to confirm any improvements in OS and QoL.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40261-023-01285-4.
Related collections
Most cited references70
- Record: found
- Abstract: found
- Article: not found
Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC
- Record: found
- Abstract: found
- Article: found
