- Record: found
- Abstract: found
- Article: found
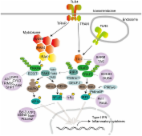
TLR2 and TLR4 mediated host immune responses in major infectious diseases: a review
2016-03-01
Read this article at
ScienceOpenPublisher
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Related collections
Most cited references151
- Record: found
- Abstract: found
- Article: found
Toll-Like Receptor Signaling Pathways
Takumi Kawasaki, Taro Kawai (2014)
- Record: found
- Abstract: found
- Article: not found
Innate immune recognition.
Charles Janeway, Ruslan Medzhitov (2002)
- Record: found
- Abstract: found
- Article: not found
Toll-like receptors and their crosstalk with other innate receptors in infection and immunity.
Taro Kawai, Shizuo Akira (2011)