- Record: found
- Abstract: found
- Article: found
Cadmium Removal from Contaminated Water Using Polyelectrolyte-Coated Industrial Waste Fly Ash
Read this article at
Abstract
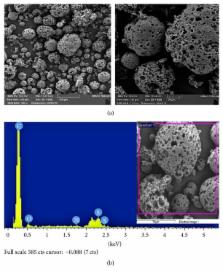
Fly ash (FA) is a major industrial waste generated from power stations that add extra cost for proper disposal. Recent research efforts have consequently focused on developing ways to make use of FA in environmentally sound applications. This study, therefore, investigates the potential ability of raw fly ash (RFA) and polyelectrolyte-coated fly ash (PEFA) to remove cadmium (Cd) from polluted water. Using layer-by-layer approach, functionalized fly ash was coated with 20 layers from 0.03% (v/v) of cationic poly(diallyldimethylammonium chloride) (PDADMAC) and anionic polystyrene sulfonate (PSS) solutions. Both surface morphology and chemical composition of the adsorbent (PEFA) were characterized using Field-Emission Scanning Electron Microscope (FE-SEM), X-Ray Diffraction (XRD), Fourier-Transform Infrared (FTIR), and X-Ray Fluorescence (XRF) techniques. The effects of pH, adsorbent dosage, contact time, initial contaminant concentration, and mixing rate of the adsorption of Cd were also studied in batch mode experiments. Results of the study revealed that a 4.0 g/L dosage of PEFA removed around 99% of 2.0 mg/L of Cd in 15 min at 150 rpm compared to only 27% Cd removal achieved by RFA under the same conditions. Results also showed that adsorption by PEFA followed both Langmuir and Freundlich models with correlation coefficients of 98% and 99%, respectively.
Related collections
Most cited references49
- Record: found
- Abstract: not found
- Article: not found
THE ADSORPTION OF GASES ON PLANE SURFACES OF GLASS, MICA AND PLATINUM.
- Record: found
- Abstract: found
- Article: not found
Organic polyelectrolytes in water treatment.
- Record: found
- Abstract: found
- Article: not found