- Record: found
- Abstract: found
- Article: found
Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases
news
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Background
The outbreak of the novel coronavirus disease,
COVID-19, caused by the new coronavirus 2019-nCoV that is now officially
designated as severe acute respiratory syndrome-related coronavirus
SARS-CoV-2, represents a pandemic threat to global public health.
1,2
Although the epicenter of the COVID-19 outbreak in December of 2019
was located in Wuhan, China, this disease has spread to more than
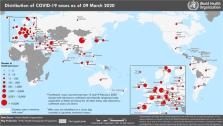
100 countries (Figure 1
) with over 100 000 confirmed cases and over 3,800 confirmed
deaths worldwide (Figure 2
) as of March 9, 2020.
3
In addition,
millions of people’s lives have been affected as a result of
mandatory isolations/quarantines. The ripple effect of the COVID-19
outbreak could potentially bring major challenges to worldwide health
systems and have far-reaching consequences on the global economy if
the spread of the virus is not effectively controlled.
1,2,4
Figure 1
Global distribution of confirmed COVID-19
cases. (Map was reproduced from WHO Coronavirus Disease (COVID-2019)
Situation Reports.
3
Used with permission
from ref (3). Copyright
2020 World Health Organization.)
Figure 2
Global trend of confirmed
COVID-19 cases and associated deaths from January 23 through March
9, 2020. (Data were obtained from WHO Coronavirus Disease (COVID-2019)
Situation Reports
3
).
Coronaviruses (CoVs) are relatively large viruses containing a single-stranded
positive-sense RNA genome encapsulated within a membrane envelope.
The viral membrane is studded with glycoprotein spikes that give coronaviruses
their crown-like appearance (Figure 3
). While coronaviruses infect both humans and animals,
certain types of animals such as bats that host the largest variety
of coronaviruses appear to be immune to coronavirus-induced illness.
5
There are four classes of coronaviruses designated
as alpha, beta, gamma, and delta. The betacoronavirus class includes
severe acute respiratory syndrome (SARS) virus (SARS-CoV), Middle
East respiratory syndrome (MERS) virus (MERS-CoV), and the COVID-19
causative agent SARS-CoV-2. Similar to SARS-CoV and MERS-CoV, SARS-CoV-2
attacks the lower respiratory system to cause viral pneumonia, but
it may also affect the gastrointestinal system, heart, kidney, liver,
and central nervous system leading to multiple organ failure.
6,7
Current information indicates that SARS-CoV-2 is more transmissible/contagious
than SARS-CoV.
8
Figure 3
Cartoon illustration
of the coronavirus structure and viral receptor ACE2 on the host cell
surface. (Image was reproduced with permission from ref (9), Nature Reviews
Microbiology 7(3), 226–236. Copyright 2009 Springer
Nature.)
The betacoronavirus genome encodes
several structural proteins, including the glycosylated spike (S)
protein that functions as a major inducer of host immune responses.
This S protein mediates host cell invasion by both SARS-CoV and SARS-CoV-2
via binding to a receptor protein called angiotensin-converting enzyme
2 (ACE2) located on the surface membrane of host cells.
9−11
A recent study also revealed that this invasion process requires S
protein priming which is facilitated by the host cell-produced serine
protease TMPRSS211. In addition, the viral genome also encodes several
nonstructural proteins including RNA-dependent RNA polymerase (RdRp),
coronavirus main protease (3CLpro), and papain-like protease (PLpro).
12,13
Upon entrance to the host cells, the viral genome is released as
a single-stranded positive RNA. Subsequently, it is translated into
viral polyproteins using host cell protein translation machinery,
which are then cleaved into effector proteins by viral proteinases
3CLpro and PLpro.
12,13
PLpro also behaves as a deubiquitinase
that may deubiquinate certain host cell proteins, including interferon
factor 3 and NF-κB, resulting in immune suppression.
13,14
RdRp synthesizes a full-length negative-strand RNA template to be
used by RdRp to make more viral genomic RNA.
The interaction
between viral S protein and ACE2 on the host cell surface is of significant
interest since it initiates the infection process. Cryo-EM structure
analysis has revealed that the binding affinity of SARS-CoV-2 S protein
to ACE2 is about 10–20 times higher than that of SARS-CoV S
protein.
10,15
It is speculated that this may contribute
to the reported higher transmissibility and contagiousness of SARS-CoV-2
as compared to SARS-CoV.
8
The prospect
also exists for discovery of therapeutic agents targeting the highly
conserved proteins associated with both SARS-CoV and SARS-CoV-2.
15−18
RdRp and 3CLpro protease of SARS-CoV-2 share over 95% of sequence
similarity with those of SARS-CoV despite the fact that these two
viruses demonstrate only 79% sequence similarity at the genome level.
15−18
On the basis of sequence alignment and homology modeling, SARS-CoV
and SARS-CoV-2 share a highly conserved receptor-binding domain (RBD),
a domain of S protein, and 76% of sequence similarity in their S proteins.
15−18
In addition, although the PLpro sequences of SARS-CoV-2 and SARS-CoV
are only 83% similar, they share similar active sites.
16
To date, there are no SARS-CoV-2-specific
antiviral agents. Researchers have been racing to find possible treatments
to save lives and produce vaccines for future prevention. To support
research and development efforts to discover effective therapeutic
and preventive agents for COVID-19, CAS, a division of the American
Chemical Society specializing in scientific information solutions,
has analyzed scientific data related to the development of therapeutic
agents and vaccines for human coronaviruses since 2003. The analyses
presented in this report are based on the CAS content collection,
a scientist-curated data collection covering published scientific
literature and patents from over 60 patent authorities worldwide.
For a subset of the analyses, both CAS and MEDLINE data were collectively
analyzed.
Scientific Literature and Patents Related to COVID-19, SARS,
and MERS
Trend in Scientific Publications Related to COVID-19
Since the outbreak of COVID-19, this new disease and its causative
virus have drawn major global attention. Scientists and physicians
worldwide have been conducting a major campaign to understand this
new emergent disease and its epidemiology in an effort to uncover
possible treatment regimens, discover effective therapeutic agents,
and develop vaccines. Figure 4
shows the total number of journal articles related to COVID-19
or SARS-CoV-2 published each week from the last week of 2019 through
the week of February 24, 2020. Over 500 journal articles were published
electronically or in print during this period, and the number of published
articles has increased each week since the week of January 13, 2020.
Although a large portion of these articles are about clinical manifestations
and treatment options, an increasing number of studies are focused
on elucidation of virus structure, virus transmission mechanisms/dynamics,
as well as identification of antiviral agents and accurate diagnostics
for virus detection. These trends reflect immense interest and desire
from the scientific community, including both academic and industrial
organizations as well as clinicians, to identify new methods to halt
the progression of this epidemic disease and to prevent infection
and transmission in the future.
Figure 4
Number of journal articles related to
COVID-19 published each week.
Notable Journal Articles Related to COVID-19 and SARS-CoV-2
Table 1
lists some
journal articles published from December 30, 2019 through February 23,
2020. These articles were selected based on collective use of factors
such as journal impact factor, citation, and type of study. For example,
the No. 8 article listed about the characterization of the SARS-CoV-2
genome has greatly facilitated the global effort to develop a vaccine
for prevention of COVID-19. Also shown in this table are journal articles
pertaining to potential antiviral drug candidates such as remdesivir, baricitinib,
and chloroquine for the treatment of this disease.
Table 1
Notable Journal Articles on COVID-19
and/or SARS-CoV-2 Published as of February 23, 2020a
no.
journal
paper title
publication date
organization
1
The New England Journal of Medicine
A novel coronavirus from patients with pneumonia in China,
2019
January 24, 2020
NHC Key Laboratory
of Biosafety, China, and National Institute for Viral Disease Control,
Chinese Center for Disease Control and Prevention, Beijing, Chinab
2
Lancet
Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China
January 24, 2020
Department of Pulmonary and Critical
Care Medicine, China-Japan Friendship Hospital, Beijing, China; NHC
Key Laboratory of Systems Biology of Pathogens and Christophe Merieux
Laboratory, Institute of Pathogen Biology, Chinese Academy of Medical
Sciences and Peking Union Medical College, Beijing, Chinab
3
The New England Journal of Medicine
Early transmission dynamics in Wuhan, China, of novel coronavirus-infected
pneumonia
January 29, 2020
Chinese Center
for Disease Control and Prevention, Beijing, China; School of Public
Health, University of Hong Kong, Hong Kong; Hubei Center for Disease
Control and Prevention, Wuhan, Hubei, Chinab
5
Journal of Virology
Receptor recognition by novel
coronavirus from Wuhan: An analysis based on decade-long structural
studies of SARS
January 29, 2020
Department
of Epidemiology, University of North Carolina, Chapel Hill, NC, USA
6
Lancet
Epidemiological
and clinical characteristics of 99 cases of 2019 novel coronavirus
pneumonia in Wuhan, China: a descriptive study
January
30, 2020
Tuberculosis and Respiratory Department, Wuhan
Jinyintan Hospital, Wuhan, China
7
The New England Journal of Medicine
First case
of 2019 novel coronavirus in the United States
January
31, 2020
The Washington State Department of Health Public
Health Laboratories, WA, USAb
8
Lancet
Genomic characterisation and epidemiology of 2019 novel coronavirus:
implications for virus origins and receptor binding
January 30, 2020
NHC Key Laboratory of Biosafety, National
Institute for Viral Disease Control and Prevention, Chinese Center
for Disease Control and Prevention, Beijing, China, Central Theater,
People’s Liberation Army General Hospital, Wuhan, China, Center
for Biosafety Mega-Science, Chinese Academy of Sciences, Beijing, Chinab
9
Lancet
Nowcasting and forecasting
the potential domestic and international spread of the 2019-nCoV outbreak
originating in Wuhan, China: a modelling study
January
31, 2020
School of Public Health, Li Ka Shing Faculty
of Medicine, University of Hong Kong, Hong Kong, Chinab
10
Nature
A new coronavirus associated with human
respiratory disease in China
February 3, 2020
Shanghai Public Health Clinical Center & School of Public
Health, Fudan University, Shanghai, Chinab
11
Nature
A pneumonia outbreak associated with a
new coronavirus of probable bat origin
February 3, 2020
Key Laboratory of Special Pathogens, Wuhan Institute of Virology,
Center for Biosafety Mega-Science, Chinese Academy of Sciences, Wuhan, Chinab
12
Lancet
Baricitinib as potential
treatment for 2019-nCoV acute respiratory disease
February
4, 2020
BenevolentAI, London, UK and Department of Surgery
and Cancer, Imperial College London, UK
13
Cell Research
Remdesivir and chloroquine effectively
inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro
February 4, 2020
State Key Laboratory of Virology,
Wuhan Institute of Virology, Center for Biosafety Mega-Science, Chinese
Academy of Sciences, Wuhan, China, and National Engineering Research
Center for the Emergency Drug, Beijing Institute of Pharmacology and
Toxicology, Beijing, Chinab
14
Emerging Microbes & Infections
RNA based
mNGS approach identifies a novel human coronavirus from two individual
pneumonia cases in 2019 Wuhan outbreak
February 5, 2020
State Key Laboratory of Virology, Modern Virology Research
Center, College of Life Sciences, Wuhan University, Wuhan, Chinab
15
The Journal of the American Medical Association
Clinical characteristics of 138 hospitalized patients with
2019 novel coronavirus-infected pneumonia in Wuhan, China
February 7, 2020
Department of Critical Care Medicine,
Zhongnan Hospital of Wuhan University, Wuhan, Hubei, China
16
Cell Host & Microbe
Genome composition and divergence of the novel
coronavirus (2019-nCoV) originating in China
February
7, 2020
National Institute for Viral Disease Control
and Prevention, China CDC, Beijing, China; Department of Microbiology,
Immunology and Molecular Genetics, University of California, Los Angeles,
USA; Center for Systems Medicine, Institute of Basic Medical Sciences
& Peking Union Medical College, Beijing, Chinab
17
Cellular & Molecular Immunology
Fusion mechanism of 2019-nCoV and fusion inhibitors targeting
HR1 domain in spike protein
February 11, 2020
Key Laboratory of Medical Molecular Virology, School of Basic
Medical Sciences, Fudan-Jinbo Joint Research Center, Fudan University,
Shanghai, China
a
Note: The publication date is the
date for electronic publication.
b
Only corresponding organization(s) is/are listed for papers published
by multiple organizations.
Distribution
of patents related to SARS and MERS
As mentioned earlier,
COVID-19 is caused by SARS-CoV-2, a new type of coronavirus in the
same genus as SARS-CoV and MERS-CoV. Viral proteins responsible for
SARS-CoV-2 entry into host cells and replication are structurally
similar to those associated with SARS-CoV. Thus, research and development
on SARS and MERS may offer insights that would be beneficial to the
development of therapeutic and preventive agents for COVID-19. This
report identified pertinent data from patents related to these two
coronaviruses. Figure 5
shows the distribution of patents in the CAS content collection
related to SARS (A) and MERS (B). The number of patents related to
SARS is almost 12 times the number related to MERS, probably because
the SARS outbreak occurred about 10 years before the MERS outbreak.
Among SARS patents, about 80% are related to the development of therapeutics,
35% are related to vaccines, and 28% are related to diagnostic agents
or methods. Because an individual patent may cover any two or more
areas, the sum of percentage values is greater than 100%. A similar
distribution pattern was also observed for patents related to MERS.
Thus, for both diseases, more patents have been devoted to the development
of therapeutic agents as opposed to diagnostic methods and vaccines.
Figure 5
Distribution
of patents related to SARS (A) and MERS (B) based on application purpose.
RESEARCH AND DEVELOPMENT IN SMALL MOLECULE
ANTIVIRAL AGENTS FOR COVID-19 AND RELATED CORONAVIRUS DISEASES
Key Proteins
and Their Roles in Viral Infection
Identification of targets
is important for identifying drugs with high target specificity and/or
uncovering existing drugs that could be repurposed to treat SARS-CoV-2
infection. Table 2
lists
potential targets, their roles in viral infection, and representative
existing drugs or drug candidates that reportedly act on the corresponding
targets in similar viruses and thus are to be assessed for their effects
on SARS-CoV-2 infection. 3CLpro and PLpro are two viral proteases
responsible for the cleavage of viral peptides into functional units
for virus replication and packaging within the host cells. Thus, drugs
that target these proteases in other viruses such as HIV drugs, lopinavir
and ritonavir, have been explored.
19
RdRp
is the RNA polymerase responsible for viral RNA synthesis that may
be blocked by existing antiviral drugs or drug candidates, such as
remdesivir.
19
Conceivably, the interaction
of viral S protein with its receptor ACE2 on host cells, and subsequent
viral endocytosis into the cells, may also be a viable drug target.
For example, the broad-spectrum antiviral drug Arbidol, which functions
as a virus-host cell fusion inhibitor to prevent viral entry into
host cells against influenza virus,
20
has
entered into a clinical trial for treatment of SARS-CoV-2.
21,22
The protease TMPRSS2 produced by the host cells plays an important
role in proteolytic processing of S protein priming to the receptor
ACE2 binding in human cells.
11
It has been
shown that camostat mesylate, a clinically approved TMPRSS2 inhibitor,
was able to block SARS-CoV-2 entry to human cells, indicating its
potential as a drug for COVID-19.
11
Table 2
Key Proteins and Their Roles during the Viral Infection
Process
target candidate
full name
role during viral infection
drug candidate
3CLpro
coronavirus main protease 3CLpro
a protease for the proteolysis of viral polyprotein into functional
units
lopinavir
19,30
PLpro
papain-like
protease PLpro
a protease for the proteolysis of viral
polyprotein into functional units
lopinavir
19,30
RdRp
RNA-dependent
RNA polymerase
an RNA-dependent RNA polymerase for replicating
viral genome
remdesivir,
19,29,32
ribavirin
16,29,31
S protein
viral spike
glycoprotein
a viral surface protein for binding to
host cell receptor ACE2
Arbidol
20,22,33
a
TMPRSS2
transmembrane protease, serine 2
a host cell-produced protease that primes S protein to facilitate its binding to ACE2
camostat mesylate
11
ACE2
angiotensin-converting enzyme 2
a viral receptor protein on
the host cells which binds to viral S protein
Arbidol
20,22,33
a
AT2
angiotensin AT2 receptor
an important effector involved in the regulation of blood pressure
and volume of the cardiovascular system
L-163491
28
a
An inhibitor of viral entry to host cells. Its direct action on S protein and ACE2
is yet to be confirmed.
ACE2 involvement with coronavirus infection is of further interest
since ACE2 is a potent negative regulator restraining overactivation
of the renin-angiotensin system (RAS) that may be involved in elicitation
of inflammatory lung disease in addition to its well-known role in
regulation of blood pressure and balance of body fluid and electrolytes.
23,24
It catalyzes degradation of angiotensin II to angiotensin (1–7).
The balance between angiotensin II and angiotensin (1–7) is
critical since angiotensin II binds to angiotensin AT1 receptor to
cause vasoconstriction, whereas angiotensin (1–7) elicits vasodilation
mediated by AT2.
25−27
Although the notion that ACE2 mediates coronavirus
invasion is largely accepted, it remains unclear how the levels or
activities of ACE2, AT1 receptors, and AT2 receptors are altered in
coronavirus-induced diseases due to the limited number of studies.
23,24
Therefore, it is yet to be determined whether some drugs or compounds
that target any of these proteins (e.g., L-163491 as a partial antagonist
of AT1 receptor and partial agonist of AT2 receptor) may alleviate
coronavirus-induced lung injury.
28
Patents and Potential Drug
Candidates Related to Key Protein Targets
The CAS content
collection contains patents related to coronavirus key proteins listed
above. Table 3
lists
the number of patents related to each protein target and associated
therapeutic compounds with a CAS Registry Number (CAS RN) reported
in these patents. CAS data show that targets 3CLpro and RdRp attracted
more attention than other targets, and more compounds with therapeutic
potential were identified for these targets, probably due to the work
done for SARS-CoV which also contains 3CLpro and RdRp.
Table 3
Key Protein Targets and Related Patents in the CAS Content Collection
and Potential Drug Candidates in CAS REGISTRY of Chemical Substances
target
no. of patents
no. of
potential drug candidates
3CLpro
49
2178
PLpro
4
189
RdRp
26
570
S protein
46
333
ACE2
5
97
AT2
2
38
Existing Drugs with Potential
Therapeutic Applications for COVID-19
Since SARS-CoV-2 is
a newly discovered pathogen, no specific drugs have been identified
or are currently available. An economic and efficient therapeutic
strategy is to repurpose existing drugs. On the basis of genomic sequence
information coupled with protein structure modeling, the scientific
community has been able to rapidly respond with a suggested list of
existing drugs with therapeutic potential for COVID-19. Table 4
provides a summary of such
drugs together with potential mechanisms of actions for their activities.
Barcitinib was proposed because of its anti-inflammatory effect and
possible ability to reduce viral entry.
35
A fixed dose of the anti-HIV combination, lopinavir–ritonavir,
is currently in clinical trials with Arbidol or ribavirin.
22
Remdesivir, developed by Gilead Sciences Inc.,
was previously tested in humans with Ebola virus disease and has shown
promise in animal models for MERS and SARS. The drug is currently being studied in
phase III clinical trials
in both China and the USA. Favipiravir, a purine nucleoside
leading to inaccurate viral RNA synthesis,
36
was originally developed by Toyama Chemical of Japan, and has recently
been approved for a clinical trial as a drug to treat COVID-19.
30
Chloroquine, an antimalarial drug, has proven
effective in treating coronavirus in China.
32
In addition to the above-mentioned, many other antiviral drugs are
also listed.
Table 4
Existing Drugs with Therapeutic Potentials
for COVID-19 (Drug Repurposing)
drug candidate
CAS RN
target
possible mechanism of action on COVID-19
disease indication
baricitinib
35
1187594-09-7
JAK kinase
a JAK inhibitor that may
interfere with the inflammatory processes
approved drug
for rheumatoid arthritis
lopinavir
19
a
192725-17-0
viral
proteases: 3CLpro or PLpro
protease inhibitors that
may inhibit the viral proteases: 3CLpro or PLpro
lopinavir
and ritonavir are approved drug combination for HIV infection
ritonavir
19,37
c
155213-67-5
darunavir
33
206361-99-1
approved drug for HIV infection
favipiravir (favilavir)
29,36
259793-96-9
RdRp
a purine nucleoside that acts as an alternate substrate leading
to inaccurate viral RNA synthesis
viral infections
remdesivir
19,29,32
a
1809249-37-3
a nucleotide analogue that may block viral nucleotide
synthesis to stop viral replication
Ebola virus infection
ribavirin
16,29−31
a
36791-04-5
RSV infection, hepatitis C, some viral hemorrhagic fevers
galidesivir
34
b
249503-25-1
hepatitis C, Ebola virus, Marburg virus
BCX-4430 (salt form of galidesivir)
34
b
222631-44-9
hepatitis C, Ebola
virus, Marburg virus
Arbidol
22,33
a
131707-23-8
S protein/ACE2d
an inhibitor that may disrupt the binding of
viral envelope protein to host cells and prevent viral entry to the
target cell
influenza antiviral drug
chloroquine
29,32
54-05-7
endosome/ACE2
a drug that can elevate endosomal pH
and interfere with ACE2 glycosylation
malarial parasite
infection
nitazoxanide
29
55981-09-4
N/A
a drug that
may inhibit viral protein expression
various helminthic,
protozoal, and viral infection-caused diarrhea
a
Drugs under clinical trials for treating COVID-19 (repurposing).
b
Drugs under clinical trials
for other virus-induced diseases.
c
Ritonavir is a pharmacokinetic profile enhancer that may potentiate
the effects of other protease inhibitors due to its ability to attenuate
the degradation of those drugs by the liver enzyme CYP3A4 and thus
is used in combination with antivirial Lopinavir.
37
d
An inhibitor of viral entry to host cells. Its direct action on S protein and ACE2
is yet to be confirmed.
Selected Patents Related
to Promising Small Molecule Drug Candidates
Table 5
shows selected patents associated
with the aforementioned potential drugs, together with patents disclosing
small molecules for treatment of SARS or MERS. The selection was based
on the presence of important terms in CAS-indexed patents as well
as the presence of the synthetic preparation role assigned by CAS
scientists during document indexing. Patent applications WO2009114512
and WO2014028756 disclose preparation of compounds active as JAK inhibitors,
one of which was later named as baricitinib and developed for reducing
inflammation in rheumatoid arthritis. Patent application JP5971830
discloses preparation of polycyclic pyridone compounds and their use
as endonuclease inhibitors. Patent applications US20160122374 and US20170071964 disclose
preparation of the nucleotide analog drug remdesivir that was later
developed as a therapeutic agent for Ebola and Marburg virus infections
(Patent US20170071964). Because of its promising results in at least
two COVID-19 patients, remdesivir has now entered into phase III clinical
trials.
Table 5
Selected Patents Associated with Potential
Drugs (Repurposing) for COVID-19 or Small Molecules for Treatment
of SARS or MERS
patent no.
priority date
title
organization
WO2009114512
20080311
Preparation of azetidine and cyclobutane derivatives as JAK
inhibitors
Incyte Corporation, USA
WO2014028756
20140220
Deuterated baricitinib
Concert Pharmaceuticals,
Inc., USA
JP5971830
20150428
Preparation of polycyclic pyridone
derivatives as cap-dependent endonuclease (CEN) inhibitors and prodrugs
thereof
Shionogi and Co., Ltd., Japan
US20160122374
20141029
Preparation of nucleosides and methods for
treating Filoviridae virus infections
Gilead Sciences, Inc., USA
US20170071964
20160916
Preparation of amino acid-containing
nucleotides and methods for treating arenaviridae and coronaviridae
virus infections
Gilead Sciences, Inc., USA
WO2007075145
20070704
Preparation of benzopyranone derivatives as
anti-coronaviral agents
Singapore Polytechnic, Singapore;
Shanghai Institute of Materia Medica Chinese Academy of Sciences,
China
WO2005021518
20050310
Preparation of 3,4-dihydro-2H-1,4-benzoxazine-2-carboxylic acid derivatives as cysLT2
receptor antagonists for treatment of respiratory diseases
Ono Pharmaceutical Co., Ltd., Japan
WO2007120160
20071025
Preparation of N-heterocyclic acetamides useful for viral inhibition
Novartis AG, USA
WO2009119167
20091001
Aniline derivative having anti-RNA
viral activity
KinoPharma, Inc., Japan
WO2013049382
20130404
Broad-spectrum antivirals against 3c or 3c-like proteases of
picornavirus-like supercluster: picornaviruses, caliciviruses and
coronaviruses
Kansas State University Research Foundation;
The Ohio State University; Wichita State University - all in USA
WO2018042343
20180308
Preparation of peptides that inhibit 3C and 3CL proteases and methods
of use thereof
GlaxoSmithKline, UK
WO2007067515
20070614
Five-membered iminocyclitol derivatives as selective and potent
glycosidase inhibitors: new structures for antivirals and osteoarthritis
therapeutics
Academia Sinica, Taiwan
Patent application WO2013049382 discloses both structures
and syntheses of compounds from various structure classes (peptidyl
aldehydes, peptidyl α-ketoamides, peptidyl bisulfite salts,
and peptidyl heterocycles), as well as certain formulation compositions,
developed to inhibit viral 3C protease or 3C-like protease (i.e.,
3CLpro).
Patent application WO2018042343 presents both preparation
methods and biological assay results for compounds capable of inhibiting
the SARS virus proteases. These compounds appeared to exhibit good
enzyme-inhibiting activity (pIC50 ≈ 7 or IC50 ≈ 0.1 μM) and antiviral activity, which
was
assessed by host cell viability using cultured human lung fibroblast
MRC-5 cells infected with a specified virus (e.g., MERS virus) expressing
the viral S protein. Drug administration routes were also mentioned
in this patent.
Small Molecule Compounds in Research and
Development with Potential Effects on Key Protein Targets for Human
Coronavirus-Induced Diseases
Besides various commercialized
antiviral drugs, there are also small molecule compounds currently
in research and development that have shown significant inhibitory
effects on many key proteins from similar coronaviruses such as SARS-CoV
and MERS-CoV (Table 6
). These drug candidates mostly inhibit viral enzymes including proteases
and components for RdRp. Since 3CLpro protease has a high level of
sequence homology between SARS-CoV and SARS-CoV-2, inhibitors against
3CLpro of SARS-CoV may also be applicable to SARS-CoV-2. Compounds,
including benzopurpurin B, C-467929, C-473872, NSC-306711 and N-65828,
which may inhibit the activity of viral NSP15, poly(U)-specific endoribonuclease,
were tested for reduced SARS-CoV infectivity in cultured cells with
IC50 of 0.2–40 μM.
38
Compound C-21 and CGP-42112A are two AT2 agonists, whereas L-163491
has dual functions as a partial agonist for AT2 receptor and a partial
antagonist of AT1 receptor. Since AT1 and AT2 are important effectors
in the RAS system to which ACE2 belongs, it has been speculated that
these compounds may be used to adjust the balance between AT1 and
AT2, which may be affected by coronavirus infection and to alleviate
viral-induced lung injury during the infection.
24
Table 6
Small Molecule Compounds in Research and Development with Therapeutic
Potential for COVID-19
CAS RN
small molecule compound
target
possible mechanism of action
on COVID-19
4431-00-9
aurine tricarboxylic acid
RNA-dependent RNA polymerase (RdRp)
an inhibitor
that may bind to viral RdRp, as tested against SARS-CoV in cell culture
16
502960-90-9
4-methyl-N-[(1S)-2-oxo-2 [[(1S,2E)-1-(2-phenylethyl)-3-(phenylsulfonyl)-2-propen-1-yl]amino]-1-(phenylmethyl)ethyl]-
1-piperazinecarboxamide
viral proteases: 3CLpro and
PLpro
an inhibitor that may disrupt the function of
3CLpro and PLpro, which was tested against SARS-CoV
16,39,40
1851279-09-8
4-(1,1-dimethylethyl)-N-[(1S)-2-oxo-2-[[(1S,2E)-1-(2-phenylethyl)-3-(phenylsulfonyl)-2-propen-1-yl]amino]-1-(phenylmethyl)ethyl]-
1-piperazinecarboxamide
1851280-00-6
4-(2-methoxyethyl)-N-[(1S)-2-oxo-2-[[(1S,2E)-1-(2-phenylethyl)-3-(phenylsulfonyl)-2-propen-1-yl]amino]-1-(phenylmethyl)ethyl]-
1-piperazinecarboxamide
223537-30-2
rupintrivir
a cysteine protease inhibitor that may disrupt
the function of 3CLpro and PLpro
41
2409054-43-7
(αR)-α-[[3-(4-chloro-2-fluorophenyl)-1-oxo-2-propen-1-yl]amino]-N-[(1R)-1-methyl-2-(2-oxo-3-pyrrolidinyl)ethyl]-
benzenepropanamide
viral proteases: 3CLpro or PLpro
an inhibitor that may disrupt the function of 3CLpro or PLpro,
as tested against SARS-CoV or MERS-CoV
39,40
452088-38-9
5-[(4-methyl-1-piperidinyl)sulfonyl]-1H-indole-2,3-dione
2409054-44-8
3-hydroperoxy-4-[2-hydroxy-3-[3-(4-hydroxyphenyl)-1-oxo-2-propen-1-yl]-6-methoxyphenyl]-2-butanone
41137-87-5
hirsutenone
992-59-6
benzopurpurin B
NSP15
(poly(U)-specific endoribonuclease)
chemical inhibitors
that may suppress viral infectivity by inhibiting endoribonuclease
NSP15, as tested against SARS-CoV in cultured cells
38
351891-58-2
C-467929
331675-78-6
C-473872
813419-93-1
NSC-306711
501444-06-0
N-65828
477775-14-7
C-21
AT2
an angiotensin AT2 receptor agonist
that may alleviate the virus-induced lung injury
24
127060-75-7
CGP-42112A
170969-73-0
L-163491
a dual-property molecule that functions
as angiotensin AT1 partial antagonist and AT2 agonist which may alleviate
the virus-induced lung injury
24
Small Molecules Identified by Structure Similarity, Lipinski’s
Rule of 5, and CAS-Indexed Pharmacological Activity and/or Therapeutic
Usage
Besides the aforementioned antiviral drugs, there may
be additional small molecule compounds with therapeutic or pharmacological
potential against viruses such as SARS-CoV and MERS-CoV. Compounds
listed in Tables 4
and 6 were subjected to a Tanimoto similarity search
in CAS REGISTRY using CAS proprietary fingerprints.a Those substances with at least
60% structural similarity
match and meeting Lipinski’s rule of 5 were identified. Table 7
lists selected compounds
that were also identified to have a pharmacological activity or therapeutic
usage role. Compound name and CAS RN are provided for each compound.
The second column lists the number of compounds that met the structure
similarity and Lipinski’s rule criteria. Although more work
remains to be done in this regard, the methodology and results mentioned
here point to a strategy that may help streamline the process of drug
discovery for COVID-19.
Table 7
Examples of Similar
Molecules with Possible Therapeutic Effects Identified by Structural
Similarity, Lipinski’s Rule of 5, and Pharmacology/Therapeutic
Role Assigned by CAS Scientists during Document Indexing
query substance name (CAS RN)
no. of substances with >60% similarity
example of selected similar substance
Registry Number of selected similar substance
ribavirin (36791-04-5)
1520
viramidine
119567-79-2
galidesivir (249503-25-1)
502
(2R,3S,5R)-5-(4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-yl)-3-hydroxy-2-pyrrolidinemethanol
1610426-50-0
(2S,4R,5S)-5-(4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-yl)-4-hydroxy-2-pyrrolidinemethanol
872534-76-4
(2R,3R,4S,5S)-5-(4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-yl)-3-hydroxy-4-methoxy-2-pyrrolidinemethanol
1610426-51-1
chloroquine (54-05-7)
21176
hydroxychloroquine
118-42-3
(±)-chloroquine diphosphate
50-63-5
chloroquine hydrochloride
3545-67-3
chloroquine
sulfate
132-73-0
favipiravir (259793-96-9)
309
6-bromo-3,4-dihydro-3-oxo-2-pyrazine-5-d-carboxamide
1476773-04-2
6-fluoro-3,4-dihydro-3-oxo-2-pyrazine-5-d-carboxamide
1492021-26-7
2-butanone, 3-hydroperoxy-4-[2-hydroxy-3-[3-(4-hydroxyphenyl)-1-oxo-2-propen-1-yl]-6-methoxyphenyl]
(2409054-44-8)
63195
xanthoangelol D
132998-83-5
BIOLOGICS FOR
CORONAVIRUS-ASSOCIATED DISEASES
Distribution of Biologics Patents Related
to SARS and MERS
The new coronavirus SARS-CoV-2 related to
SARS and MERS viruses is causing serious and ongoing epidemiological
problems around the world. Since there is limited clinical and basic
research information at this time, treatment options for COVID-19
currently comprise investigational drugs and management of symptoms.
As biologics have the potential to broaden the spectrum of the treatment
options for coronavirus-induced diseases, leveraging prior knowledge
and practices used to address the SARS and MERS outbreaks provides
a practical strategy for developing new target-specific therapeutic
agents for SARS-CoV-2. To this end, an analysis of biologics from
patents contained in the CAS content collection was performed. The
patent analysis included information related to therapeutic antibodies,
cytokines, interfering and other therapeutic RNAs, and vaccines for
potential treatment and/or prevention of SARS-related diseases from
patents published from 2003 to the present. Figure 6
shows more than 500 patents that disclose
the use of these four biologics classes to treat and prevent SARS
and MERS. Of these patents, vaccine development was the largest class
(363), followed by therapeutic antibodies (99), interfering RNAs (35),
and cytokines (22). Given the indispensable role of vaccines in viral
disease prevention, detailed analysis of vaccines will be presented
in a later section.
Figure 6
Distribution of biologics patents related to SARS and
MERS.
Antibodies
Ninety-nine
patents containing information about antibodies with therapeutic and/or
diagnostic potential for SARS and MERS were identified. Of these,
61 patents claimed preparation of SARS-specific antibodies (23), MERS-specific
antibodies (17), or antibodies with diagnostic application (21). Similar
to SARS-CoV, the receptor-binding domain (RBD) in the S protein of
SARS-CoV-2 binds to human ACE2 receptor in order to gain access into
host cells.
42
In viral infection, the S
protein, but not the other structural proteins, M, E, and N in SARS-CoV,
elicits an immune response.
43
Table 8
shows the target
analysis of patents related to development of therapeutic antibodies
for SARS. Over 90% of these antibodies are directed against S protein
including its RBD. The data indicate that the S protein is a putative
target for SARS-CoV-2 antibody development.
Table 8
Target
Analysis of Patents on Developing Therapeutic Antibodies for SARS
patent number
antigen of SARS antibody
patent title
organization
priority date
EP2112164
lipid attachment signals or GPI
Antiviral peptides linked to a lipid attachment signals or
GPI against enveloped virus such as HIV, avian flu, SARS or Ebola
virus
Institute Pasteur of Shanghai
20080229
WO2009128963
spike protein
Cross-neutralizing human monoclonal antibodies to SARS-CoV
and methods of use thereof
Institute for Research In
Biomedicine
20080117
WO2009128963
spike protein
Cross-neutralizing human monoclonal
antibodies to spike protein of SARS coronavirus and methods of use
thereof
Humab, LLC
20080117
WO2008035894
viral infection
Preparation of antiviral antibody 3D8 fragments and their use in treatment
of viral infection
Sung Kyun Kwan University; Ajou University;
Invitroplant Co., Ltd.
20060919
WO2008060331
spike protein
Antibodies
to SARS coronavirus
Amgen Inc.
20060519
WO2007044695
spike protein
Neutralizing monoclonal anti-spike protein antibodies for diagnosis
and treatment of SARS-coronavirus-associated disease and screening
of vaccine or anti-SARS agent
Dana-Farber Cancer Institute
20051007
CN1911963
RBD
of S protein
Method for
preparing neutralizing monoclonal antibody against severe acute respiratory
syndrome coronavirus and its application
Chinese Academy
of Sciences
20050810
CN1903878
spike protein
Fab fragment of human antibody
IgG against SARS coronavirus
Fudan University
20050726
WO2006095180
S2 protein
Human monoclonal antibodies against SARS-associated
coronavirus and treatment of patients with SARS
Ultra
Biotech Ltd.; University of California
20050310
WO2006086561
spike protein
Neutralizing monoclonal antibodies against severe acute respiratory
syndrome-associated coronavirus
New York Blood Center,
Inc.
20050208
CN1664100
spike protein
Preparation of heavy chain and
light chain variable regions of anti-SARS coronavirus antigen antibodies
and their diagnostic and therapeutic uses thereof
Chen
Zhinan
20050204
CN1660912
IL-8
Sequences
of monoclonal antibodies against human interleukin 8 and therapeutic use
Ye Qingwei
20041208
WO2006051091
spike protein
Compositions against SARS-coronavirus
and uses thereof
Crucell Holland BV
20041111
WO2006051091
spike protein
Compositions against SARS-coronavirus comprising at least two
immunoglobulins reacting with non-competing epitopes, and therapeutic
and diagnostic uses thereof
Crucell Holland BV
20041111
CN1673231
spike
protein
Monoclonal antibody of SARS coronavirus N protein and its use in treatment of SARS
virus
infections
Chinese Academy of Sciences
20040715
US20060240551
spike
protein
Neutralizing monoclonal antibodies against severe
acute respiratory syndrome-associated coronavirus
New
York Blood Center, Inc.
20040602
WO2005054469
spike protein
Anti-SARS-coronavirus
monoclonal antibodies, and diagnostic, therapeutic and vaccine preparation
uses
Health Canada
20031205
WO2005060520
spike protein
Antibodies specific to SARS-CoV spike protein for diagnosis and therapy
of SARS and for screening of epitopic vaccines or anti-SARS therapeutics
Dana-Farber Cancer Institute, Inc.
20031125
US20050106563
spike protein
Epitope profiles of SARS coronavirus for use in antigen detection,
antibody production, and defense against infection
Genesis
Biotech Inc.
20030908
US20050069869
spike protein
SARS coronavirus codon-optimized
sequences for spike (S) protein expression, anti-S human monoclonal antibodies, and
therapeutic and diagnostic uses
thereof
University of Massachusetts
20030804
WO2005012360
S and N proteins
Antibody binding molecules
specific for SARS coronavirus
Crucell Holland BV
20030722
CN1566155
S, N, and M proteins
Antibody library-derived
human monoclonal anti-SARS virus antibodies for treating severe acute
respiratory syndrome
Igcon Therapeutics Co., Ltd.; Genetastix
Corporation
20030710
WO2005007671
spike protein
Compositions and methods for treating
SARS using peptides derived from SARS virus E2 N-terminal-alpha helix
or C-terminal-alpha helix and related monoclonal antibody
Epitomics, Inc.
20030429
An additional 38 patents contained
information pertaining to other types of antiviral antibodies that
were useful for SARS and MERS therapies. These included neutralizing
antibodies or antibodies designed to target proteins such as IL-6/IL-6R,
TLR3 (Toll-like receptor 3), CD16, ITAM (immunoreceptor tyrosine-based
activation motif), DC-SIGN (dendritic cell-specific intercellular
adhesion molecule-grabbing nonintegrin), ICAM-3 (intercellular adhesion
molecule 3), or IP-10/CXCL10 (interferon γ-inducible protein
10). Cytokine storm has been reported to correlate with disease severity
in SARS-CoV-2 infection. Patients admitted to an ICU had higher concentrations
of proinflammatory cytokines and chemokines, particularly G-CSF, IP-10/CXCL10,
MCP1 (monocyte chemoattractant protein 1), and TNFα, as well
as elevated cytokines from T helper 2 cells such as IL-4 and IL-10.
44
Patent application WO2005058815 discloses human
anti-IP-10 antibodies, including bispecific molecules and immunoconjugates
that bind to IP-10 with high affinity, for treating inflammation,
autoimmune disease, neurodegenerative disease, bacterial infection,
and viral infection. Patent application WO2017095875 discloses the
preparation of human antibodies and immunoconjugates specifically
targeting chemokine IP-10, including an anti-IP-10 antibody shown
to suppress free serum IP-10 in about 3 days at 0.5 mg/kg and in approximately
10 days at 10 mg/kg in Cynomolgus macaques.
In addition, DC-SIGN/CD209,
a type II transmembrane adhesion molecule with C-type lectin function,
is mainly expressed on interstitial dendritic cells and lung alveolar
macrophages.
45
DC-SIGN functions as an
entry cofactor in transferring SARS-CoV to susceptible cells such
as pneumocytes.
46
Patent application WO200505824
claims the production of a humanized anti-DC-SIGN antibody that interfered
with the interaction of DC-SIGN with its receptor, ICAM-3. The antibody
effectively blocked viral binding, infection, and transmission for
viral infections/diseases, including SARS.
Cytokines
Cytokines are low-molecular-weight
proteins that act as chemical signals in the immune response to pathogen
invasion. The production of various cytokines in response to an invading
pathogen such as a virus contributes to the host organism’s
ability to eliminate the pathogen. Specific types of cytokines, including
chemokines, interferons (IFNs), interleukins, and lymphokines, have
been reported and characterized in the literature over the past 40
years. By early 2020, the CAS Lexicon contained over 700 terms for
specific types of cytokines associated with 76 724 documents,
including 11 837 patents.
During a viral infection, the
most prominent cytokines produced are IFNs, which interfere with viral
replication. IFNs are classified as type I (IFN-α, IFN-β,
IFN-δ, IFN-ε, IFN-κ, IFN-ν, IFN-τ, and
IFN-ω), type II (IFN-γ), or type III (IFN-λ) based
on the receptor complex used for signaling as well as sequence homology.
47
Because of their ability to interfere with viral
replication, interferons and interferon fusion proteins have been
utilized as therapeutic agents for treatment of viral infections for
the past 20 years. A few patents disclosing these proteins and their
use for treating SARS are described below.
rSIFN-co
Patent
applications WO2011072487 and WO2016180335 describe the cloning of
a recombinant interferon (rSIFN-co, CAS RN 2043378-94-3) as well as
a method for determining its potency that was effective for treating
various viral infections/diseases, including SARS. The invention relates
that rSIFN-co has an identical amino acid sequence to Infergen (118390-30-0),
but it has an altered spatial conformation and different biological
potency. The rSIFN-co not only has an antiviral activity that is 20
times stronger than the clinically available interferon, but also
has significantly stronger antitumor properties against breast cancer
and cervical cancer than other recombinant human α-interferons.
The invention further relates that rSIFN has reduced toxic and side
effects and can be safely used in large doses (each dose can be >10
million IU), making it possible to successfully treat some viral diseases
or tumors that require large doses of interferon.
IFN-ω
Patent application WO2004096852 discloses the amino acid sequence
for recombinant human interferon ω (rhIFN-ω) (RN 791910-34-4)
that was shown to have an anti-SARS viral activity similar to that
of IFN-β. IFN-ω effectively decreased disease severity
and inhibited proliferation of coronavirus strain BJ01 in monkeys.
IL-28A (IFN-λ2), IL-28B (IFN-λ3), and IL-29 (IFN-λ1)
Variants
Patent application WO2005097165 claims a method
for treating SARS viral infection using IL-28A, IL-28B, and IL-29
cysteine variants conjugated to polymers (e.g., polyethylene glycol)
and discloses the amino acid and nucleic acid sequences for these
cysteine variants. Of these variants, MetIL-29C172S-PEG (RN 867228-40-8)
was specifically shown to inhibit viral replication.
Interferon-Human
Serum Albumin Fusion Protein
Patents applications US20090053173
and CN101942026 both disclose long-lasting fusion proteins (HSA-IFN)
with each of them being composed of an interferon fused with human
serum albumin-binding peptide for treatment of a wide range of diseases,
including SARS. Specific HSA-IFN fusion proteins were constructed
using five different interferons (IFN-α1b, IFN-α2b, IFN-β,
IFN-ω, IFN-γ) with corresponding CAS RNs 1122730-20-4,
1122730-23-7, 1122730-25-9, 1122730-27-1, and 1122730-29-3, respectively.
These HSA-IFN fusion proteins significantly lengthened the plasma
half-life of interferons (e.g., from 10 h to 12 days for HSA-IFN-α2b)
due to slower free interferon release into the plasma and thus may
prolong the effects of interferon for each injection.
RNA Therapies
RNA interference (RNAi) is a biological process wherein small complementary
RNA duplexes target and neutralize specific mRNA molecules,
resulting in inhibition of gene expression or genetic translation.
Interfering RNAs include microRNAs and small interfering RNAs (siRNAs)
that are generally about 21–25 nucleotides in length. Short
hairpin RNAs (shRNAs) are artificial synthetic RNA molecules designed
to fold into a tight hairpin conformation that allows them to silence
their target genes, and can serve as precursors of siRNAs. The expression
of shRNAs in cells is typically accomplished by their delivery via
plasmids or viral or bacterial vectors.
48
Although microRNAs are noncoding and naturally found in plants,
animals, and some viruses, synthetic versions are currently being
used to silence a variety of genes.
49
The
ability to chemically synthesize modified analogues of microRNAs as
well as siRNAs, which are capable of altering disease-related gene
expression or inhibiting pathogen gene expression, has created a host
of new therapeutic options.
50
In
contrast to the microRNAs and siRNAs, antisense RNAs are single-stranded
RNAs which are naturally occurring or synthetic and usually around
19–23 nucleotides in length with a sequence complementary to
that of a protein coding mRNA, allowing it to hybridize and block
protein translation.
48
Since the
discovery of RNAi in the late 1990s, it has become a well-known method
for silencing/suppressing target genes associated with virulence and
pathogenesis. Thirty-five patents in the CAS content collection disclose
the use of RNAi in treating SARS, with 28 patents using siRNA molecules,
three patents using antisense oligonucleotides, two patents using
RNA aptamers, one patent using a ribozyme, and one patent using a
microRNA inhibitor. Supporting Information Table S1 provides a high-level view of
these 35 patents including
the specific RNAi targets. A few of these patents are further discussed
below.
siRNAs Targeting Coronavirus Proteins M, N, or E
Patent
application CN101173275 discloses two double-stranded RNAs (dsRNAs)
designed to specifically target two separate regions of the SARS protein
M mRNA. The siRNA-M1 sequences targeting the 220–241 region
of protein M mRNA correspond to CAS RNs 1023405-01-7 and 1023405-02-8,
while siRNA-M2 sequences targeting the 460–480 region correspond
to CAS RNs 1023405-03-9 and 1023405-04-0. The interference efficiency
of these two siRNAs on SARS M protein gene expression was greater
than 70%.
Table 9
Representative siRNA Data from Chinese
Patent CN1648249
siRNA
sense strand (CAS RN)
antisense strand (CAS RN)
No. 8*
5′-cgucgcagcguguaggcacua-3′
5′-cagugccuacacgcugcgacg-3′
(RN 874840-18-3)
(RN 874840-32-1)
No. 51*
5′-aacgguuuacgucuacucgca-3′
5′-cgcgaguagacguaaaccguu-3′
(RN 874840-19-4)
(RN 874840-47-8)
No. 56*
5′-aacguacugccacaaaacagc-3′
5′-acuguuuuguggcaguacguu-3′
(RN 874840-20-7)
(RN 874840-46-7)
siRNAs Targeting Replicase
and RNA Polymerase Region
Table 10
Representative
siRNA Data from US20050004063
siRNA
sense strand
CAS
RN
target region or gene
SARSi-1
5′-gugaacucacucgugagcuctt-3′
821121-35-1
512–531 bp of replicase
A1 region
SARSi-2
5′-guacccucuugauugcauctt-3′
821121-36-2
586–604 bp of replicase
A1 region
SARSi-3
5′-gagucgaagagaggugucutt-3′
821121-37-3
916–934 bp of replicase
A1 region
SARSi-4
5′-gcacuugucuaccuugaugtt-3′
821121-38-4
1194–1213 of replicase
A1 region
SARSi-5
5′-ccuccagaugaggaagaagtt-3′
821121-39-5
3028–3046 bp of replicase
A region
SARSi-6
5′-gguguuuccauuccaugugtt-3′
821121-40-8
5024–5042 bp of replicase
A region
SARSi-7
5′-cacugauuccguucgagauctt-3′
821121-41-9
S gene
SARSi-8
5′-cguuucggaagaaacagguactt-3′
821121-42-0
E gene
SARSi-9
5′-caagccucuucucgcuccuctt-3′
821121-43-1
N gene
SARSi-10
5′-guggcuuagcuacuucguugtt-3′
821121-44-2
M gene
SARSi-11
5′-ugcuugcugcugucuacagtt-3′
821121-45-3
M gene
The authors demonstrated that SARSi-2,
SARSi-3, SARSi-4, and SARSi-7-11 inhibited coronavirus infection and
replication in FRhk-4 cells. SARSi-4 was the most effective with nearly
complete inhibition, followed by SARSi-2 and SARSi-3.
Patent
application CN1569233 discloses siRNAs, shown in Table 11
, that target SARS genes encoding
RNA-dependent RNA polymerase, helicase, nucleoprotein N, and proteolytic
enzymes. These siRNAs were able to inhibit or kill 50–90% of
the SARS virus BJ01 strain, with the proteolytic enzyme-targeting
siRNAs being the most effective.
Table 11
Representative siRNA
Data from CN1569233
sequence
CAS RN
gene target
% inhibition of
SARS virus
5′-caucauccggugaugcuac-3′
872062-80-1
RNA-dependent
RNA polymerase
∼50
5′-uaguguauacggcaugcuc-3′
872062-81-2
helicase
∼70
5′-gugcgugcagacgguucgu-3′
872062-82-3
nucleoprotein N
∼95
5′-cguagucgcgguaauucaa-3′
872067-98-6
proteolytic enzyme
∼90
RNA Aptamers
Two Korean patents
describe the use of RNA aptamers for inhibition of SARS viruses. Patent
application KR2009128837 identifies RNA aptamers as anti-SARS agents
capable of binding to and inhibiting the double-stranded DNA unwinding
of the SARS virus helicase. Related patent application KR 2012139512
describes RNA aptamers with distinct affinity for the nucleocapsid
of SARS-CoV for potential pharmaceutical use.
Ribozymes
Patent application JP2007043942 describes a therapeutic RNA/DNA
chimeric ribozyme designed to recognize and cleave conserved common
regions and regions with loop structures in the genes of coronaviruses,
including SARS. This ribozyme specifically recognizes the GUC in viral
genes with loop conformations.
Antisense Oligonucleotides
Antisense oligonucleotides have also been developed to reduce the
severity of SARS virus infections and to prevent or treat SARS virus-associated
disease, to detect the virus in human samples, and to diagnose SARS
virus-associated diseases. Patent application WO2005023083 published
by Ionis Pharmaceuticals describes hybrid DNA/RNA antisense oligonucleotides
designed to disrupt the pseudoknot in the frameshift site of the SARS
coronavirus RNA. In addition to directly targeting the virus, antisense
oligonucleotides may be used to target disease-related proteins involved
in the inflammatory process.
Vaccines
It is
crucial to develop safe and effective vaccines to control the COVID-19
pandemic, eliminate its spread, and ultimately prevent its future
recurrence. Since the SARS-CoV-2 virus shares significant sequence
homology with two other lethal coronaviruses, SARS and MERS, the vaccines
identified in these patents related to SARS and MERS viruses could
potentially facilitate the design of anti-SARS-CoV-2 vaccines.
Distribution
of Patents Related to SARS and MERS among Vaccine Types
Antiviral
vaccines generally fall into one of the following types: inactive
or live-attenuated viruses, virus-like particle (VLP), viral vectors,
protein-based, DNA-based, and mRNA-based vaccines. There are 363 patents
in the CAS content collection related to vaccine development to prevent
viral disorders/diseases, including SARS and MERS. Of these, 175 patents
disclose vaccines for non-coronaviruses that may have relevance to
SARS and MERS, while 188 patents are directly associated with anti-SARS
and anti-MERS vaccines with a demonstrated immune response. Supporting
Information Table S2 contains additional
information on these SARS/MERS vaccine-related patents.
Figure 7
reveals the distribution
of patents among these vaccine types related to SARS and MERS. As
can be seen, 15 patents disclose information about inactive and live-attenuated
virus vaccines, 28 patents describe DNA vaccines, 21 patents disclose
information on viral vector vaccines, 13 patents disclose information
on VLP vaccines, and three patents are focused on mRNA vaccines.
Figure 7
Distribution of vaccine-related patent
associated to SARS and MERS.
It was reported that viral S protein subunit vaccines produced higher
neutralizing antibody titers and more complete protection than live-attenuated
SARS-CoV, full-length S protein, and DNA-based S protein vaccines.
51
Unsurprisingly, about half of the patents focused
on protein vaccines comprising the S protein subunit vaccine and vaccines
specifically targeting the receptor binding domain (RBD) of the S1
subunit of the viral S protein. Collectively, S protein/gene is the
preferred target site in SARS/MERS vaccine development, and the same
strategy can be potentially useful in developing SARS-CoV-2 vaccines.
A condensed report on several patents that describe vaccines for generating
immunity to SARS and MERS follows.
Attenuated Virus Vaccines
Patent application US20060039926
discloses live attenuated coronavirus or torovirus vaccines. Introduction
of a mutation (Y6398H) into the Orf1a/b polyprotein (p59/nsp14/ExoN)
was shown to completely attenuate virulence of mouse coronavirus (MHV-A59).
The attenuated MHV virus exhibited reduced replication in mice at
day five following intracerebral inoculation.
DNA-Based Vaccines
Patent application WO2005081716 discloses compositions and methods
for inducing/enhancing immune responses, particularly antigen-specific
CD8+ T cell-mediated responses, against antigens of the SARS coronavirus.
An enhancement of the immune response involving particularly cytotoxic
T cell immune responses is induced in vivo by chimeric nucleic acids
that encode an endoplasmic reticulum chaperone polypeptide (e.g.,
calreticulin) linked to at least one antigenic polypeptide or peptide
from SARS-CoV. Using gene gun delivery of DNA-coated gold particles,
vaccination of mice against a calreticulin–nucleocapsid fusion
protein resulted in potent nucleocapsid-specific humoral and T cell-mediated
immune responses. Vaccinated animals were capable of significantly
reducing the titer of a challenging vaccinia vector expressing the
N protein of the SARS virus.
Patent application WO2015081155
discloses immunogens, which comprise consensus proteins derived from
the MERS-CoV spike protein, for use in DNA-based vaccines targeting
MERS-CoV. The consensus spike protein significantly induced both humoral
and cellular immune responses, including increased titers of IgG and
neutralizing antibodies. The induced cellular immune response involved
increased CD3+CD4+ and CD3+CD8+ T cell responses that produced IFN-γ,
TNF-α, IL-2, or both IFN-γ and TNF-α. On March 3,
2020, Inovio Pharmaceutical, Inc. announced they had designed the
DNA vaccine called INO-4800 to be planned for human trials in the
United States in April.
57
Protein-Based
Vaccines
Patent application WO2010063685 by GlaxoSmithKline
(GSK) discloses a vaccine capable of provoking a protective immune
response against SARS. The vaccine comprises an S protein immunogen
and an oil-in-water emulsion adjuvant. An engineered ectodomain immunogen
(soluble S protein), in combination with the emulsion adjuvant, GSK2,
induced high levels of anti-SARS-CoV IgG2a or IgG2b antibody responses
and neutralizing antibody responses in animal models. In late February
2020, GSK announced a collaboration with Chinese firm Clover Biopharmaceuticals
to assess a coronavirus (COVID-19) vaccine candidate.
52
This collaboration will involve the use of Clover’s
protein-based coronavirus vaccine candidate (COVID-19 S-Trimer) with
GSK’s adjuvant system. By applying their Trimer-Tag technology,
Clover has manufactured an S-Trimer subunit vaccine using a rapid
mammalian cell culture-based expression system. The Trimer-Tag is
an advanced drug development platform, which enables the production
of novel, covalently trimerized fusion proteins that can better target
previous undruggable pathways.
Patent application US20070003577
discloses immunogenic compositions and vaccines associated with the
S protein of SARS coronavirus. A TriSpike SARS coronavirus vaccine
was prepared from a recombinant full-length trimeric S protein. The
recombinant protein was shown to (1) exhibit native antigenicity as
shown by reactivity with convalescent SARS patient sera; (2) exhibit
specific binding to soluble ACE2 receptor; (3) promote antibody-dependent
viral entry in otherwise refractory human Raji B cells; and (4) elicit
protection against a challenge infection in an animal model.
Patent application US20060002947 (Antigen Express, Inc., a subsidiary
of Generex) discloses the preparation of hybrid peptides composed
of three elements, including (a) an invariant chain (Ii) key peptide
for antigen presentation enhancing activity, (b) a chemical structure
linking the Ii to the antigenic epitope, and (c) an antigenic epitope
that binds to a MHC class II molecule. The methodology was used to
create Ii-Key/MHC II SARS hybrids. Recently, Generex announced that
it is developing a COVID-19 vaccine following a contractual agreement
with a Chinese consortium comprised of China Technology Exchange,
Beijing Zhonghua Investment Fund Management, Biology Institute of
Shandong Academy of Sciences, and Sinotek-Advocates International
Industry Development. The company will utilize its Ii-Key immune system
activation technology to produce a COVID-19 viral peptide for human
clinical trials.
53
Virus-like Particle Vaccines
In 2015, patent application WO2015042373 by Novavax disclosed an
immunogenic composition composed of MERS-CoV nanoparticle VLPs containing
at least one trimer of a S protein, produced by baculovirus overexpression
in Sf9 cells. This VLP preparation induced a neutralizing antibody
response in mice and transgenic cattle, when administered along with
their proprietary adjuvant Matrix M (RN 1235341-17-9). In addition,
preparations of sera from vaccinated cattle (SAB-300 or SAB-301) were
injected into Ad5-hDPP4 transduced BALB/c mice prior to challenge
with MERS-CoV. Both SAB-300 and SAB-301 were able to protect these
mice from MERS-CoV infection with a single prophylactic injection.
Novavax announced on February 26, 2020
54
that it was beginning animal testing on potential COVID-19 vaccine
candidates due to their previous experiences working with other coronaviruses,
including both MERS and SARS. Their COVID-19 candidate vaccines targeting
the S protein of SARS-CoV-2 were developed using their recombinant
nanoparticle vaccine technology along with their proprietary adjuvant
Matrix-M.
mRNA-Based Vaccines
The potential advantages of an
mRNA approach to prophylactic vaccines include the ability to mimic
natural infection to stimulate a more potent immune response as well
as the ability to combine multiple mRNAs into a single vaccine. Patent
application WO2017070626 by Moderna discloses mRNA vaccines composed
of mRNAs encoding antigenic viral full-length S, S1, or S2 proteins
from SARS-CoV and MERS-CoV virus, formulated in cationic lipid nanoparticles.
They show that mice vaccinated with mRNA encoding coronavirus full-length
S protein generated much higher neutralizing antibody titers compared
to mRNA encoding the S protein S2 subunit. New Zealand white rabbits
immunized with MERS-CoV mRNA vaccine encoding
the full-length S protein reduced more than 90% of the viral load
in the lungs of the rabbits and induced a significant amount of neutralizing
antibody against MERS-CoV. Moderna announced on February 24, 2020
55
that it has released the first batch of mRNA-1273
against SARS-CoV-2 for use in humans, prepared using methods and strategies
outlined in their previous patents. Vials of mRNA-1273 have been shipped
to the National Institute of Allergy and Infectious Diseases (NIAID),
a division of the National Institutes of Health (NIH), to be used
in the planned Phase 1 study in the United States. Moderna reports
that mRNA-1273 is an mRNA vaccine targeting a prefusion-stabilized form of the S protein
associated
with SARS-CoV-2, which was selected by Moderna in collaboration with
investigators at the NIAID Vaccine Research Center. Manufacture of
this batch was funded by the Coalition for Epidemic Preparedness Innovations.
Patent application WO2018115527 describes vaccines comprising mRNA
encoding at least one antigen of a MERS coronavirus, preferably a
S protein or a S protein fragment (S1), an envelope protein (E), a
membrane protein (M), or a nucleocapsid protein (N), all of which
were effective in inducing an antigen-specific immune response. Intradermal
administration into mice of a lipid nanoparticle (LNP)-encapsulated
mRNA mixture encoding MERS-CoV S proteins was shown to result in translation
in vivo and induction of humoral immune responses.
SUMMARY AND PERSPECTIVES
This report provides an overview of published information on global
research and development of coronavirus-related therapeutic agents
and preventive vaccines based on the extensive CAS content collection,
with a focus on patents. It includes an overview of coronavirus morphology,
biology, and pathogenesis with a particular focus on antiviral strategies
involving small molecule drugs, as well as biologics targeting complex
molecular interactions involved in coronavirus infection and replication.
The drug-repurposing effort summarized in this report is focused primarily
on agents currently known to be effective against other RNA viruses
including SARS-CoV, MERS-CoV, influenza, HCV, and Ebola as well as
anti-inflammatory drugs. The potential impact of biologics for treatment
of coronavirus infections is promising and includes a wide variety
of options including bioengineered and vectored antibodies, cytokines,
and nucleic acid-based therapies targeting virus gene expression as
well as various types of vaccines.
The information provided
in this report provides a strong intellectual groundwork for support
of ongoing research and development for discovery and development
of therapeutic agents and vaccines for treatment of COVID-19 and coronavirus-related
diseases. Because of limited space, this report devotes minimal attention
to current efforts involved in advancing more efficient and accurate
COVID-19 diagnosis methods and products.
Novel infectious diseases
resulting from RNA viruses subject to mutation and genetic recombination,
as well as cross-species transmission, will continue to present a
serious global health threat, as exemplified by COVID-19. Despite
two former major outbreaks of coronavirus infections causing the SARS
and MERS respiratory illnesses, the world remains underprepared to
effectively manage the current COVID-19 outbreak, as evidenced by
the fact that COVID-19 has resulted in thousands of deaths worldwide.
A concerted effort to develop effective drugs and vaccines against
existing and potential future coronavirus infections and other highly
pathogenic virus outbreaks is necessary to reduce overwhelming impacts
on human life and worldwide healthcare systems. Given the costly and
arduous process involved with clinical drug development, the outbreak
of COVID-19 further highlights the value of developing relatively
broad-spectrum antiviral drugs and the importance of applying innovative
approaches such as artificial intelligence to facilitate drug discovery.
Given the lengthy process of new drug development, the current strategy
of drug repurposing has become one of the chosen solutions for immediate
treatment of SARS-CoV-2 infected individuals. Long-term drug development
goals for the pharmaceutical industry include identification of inhibitors
aimed at the replication or infection processes associated with SARS-CoV-2
or other related coronaviruses, as well as the symptomatic results
of their infections leading to severe disease and/or death. The summarized
lists, contained in this report, of small molecule compounds, and
additional descriptions of biologics with properties suitable for
inhibiting several key coronavirus proteins, could serve as information
starting points for drug development. Since vaccines are crucial for
prevention of coronavirus-related epidemic diseases in the future,
it is reassuring that a number of innovative strategies are already
being deployed. Four MERS coronavirus DNA vaccine candidates began
phase 1 clinical trials in September of 2019,
56
and Moderna Inc. released its first batch of mRNA-1273 in February
of 2020, which is an mRNA vaccine against SARS-CoV-2 ready for phase
1 study in the United States.
55
Additional
collaboration in the areas of antiviral discovery processes and clinical
trial performance will enhance patients’ access to drug candidates
with improved therapeutic potential and ideally reduce the amount
of time required to bring these drugs to market. The abundance of publications and
the
rapid publication rate associated with the SARS-CoV-2 virus-related
disease outbreak, as illustrated in this report, are indicative
of the intense effort by research institutes and pharmaceutical
industries to address both molecular mechanisms and therapeutic routes
useful for treating current and future coronavirus outbreaks.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China
Chaolin Huang, Yeming Wang, Xingwang Li … (2020)
- Record: found
- Abstract: found
- Article: not found
A Novel Coronavirus from Patients with Pneumonia in China, 2019
Na Zhu, Dingyu Zhang, Wenling Wang … (2020)
- Record: found
- Abstract: found
- Article: not found
