- Record: found
- Abstract: found
- Article: found
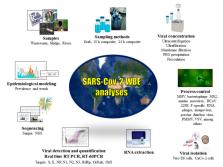
Wastewater-Based Epidemiology (WBE) and Viral Detection in Polluted Surface Water: A Valuable Tool for COVID-19 Surveillance—A Brief Review
Read this article at
Abstract
SARS-CoV-2 is the causative agent of the current COVID-19 pandemic. Disease clinical manifestations range from asymptomatic to severe multiple organ damage. SARS-CoV-2 uses ACE2 as a cellular receptor, which is abundantly expressed in the small intestine, allowing viral replication in the gastrointestinal tract. Viral RNA has been detected in the stool of COVID-19 patients and viable viruses had been isolated in some of these samples. Thus, a putative role of SARS-CoV-2 fecal-oral transmission has been argued. SARS-CoV-2 is shed in human excreta and further disposed in the sewerage or in the environment, in poor basic sanitation settings. Wastewater-based epidemiology (WBE) is a valuable population level approach for monitoring viral pathogens and has been successfully used in different contexts. This review summarizes the current global experience on SARS-CoV-2 WBE in distinct continents and viral detection in polluted surface water. The advantages and concerns of this strategy for SARS-CoV-2 surveillance are discussed. Outcomes suggest that WBE is a valuable early warning alert and a helpful complementary surveillance tool to subside public health response, to tailor containment and mitigation measures and to determine target populations for testing. In poor sanitation settings, contaminated rivers could be alternatively used as a source for environmental surveillance.
Related collections
Most cited references117
- Record: found
- Abstract: found
- Article: found
A pneumonia outbreak associated with a new coronavirus of probable bat origin
- Record: found
- Abstract: found
- Article: found
A new coronavirus associated with human respiratory disease in China
- Record: found
- Abstract: found
- Article: not found