- Record: found
- Abstract: found
- Article: found
A population-based resource for intergenerational metabolomics analyses in pregnant women and their children: the Generation R Study

Read this article at
Abstract
Introduction
Adverse exposures in early life may predispose children to cardio-metabolic disease in later life. Metabolomics may serve as a valuable tool to disentangle the metabolic adaptations and mechanisms that potentially underlie these associations.
Objectives
To describe the acquisition, processing and structure of the metabolomics data available in a population-based prospective cohort from early pregnancy onwards and to examine the relationships between metabolite profiles of pregnant women and their children at birth and in childhood.
Methods
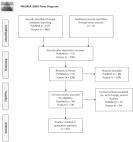
In a subset of 994 mothers-child pairs from a prospective population-based cohort study among pregnant women and their children from Rotterdam, the Netherlands, we used LC–MS/MS to determine concentrations of amino acids, non-esterified fatty acids, phospholipids and carnitines in blood serum collected in early pregnancy, at birth (cord blood), and at child’s age 10 years.
Results
Concentrations of diacyl-phosphatidylcholines, acyl-alkyl-phosphatidylcholines, alkyl-lysophosphatidylcholines and sphingomyelines were the highest in early pregnancy, concentrations of amino acids and non-esterified fatty acids were the highest at birth and concentrations of alkyl-lysophosphatidylcholines, free carnitine and acyl-carnitines were the highest at age 10 years. Correlations of individual metabolites between pregnant women and their children at birth and at the age of 10 years were low (range between r = − 0.10 and r = 0.35).
Conclusion
Our results suggest that unique metabolic profiles are present among pregnant women, newborns and school aged children, with limited intergenerational correlations between metabolite profiles. These data will form a valuable resource to address the early metabolic origins of cardio-metabolic disease.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found