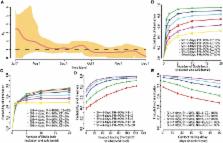
Introduction In March 2009 H1N1pdm influenza emerged in Mexico and started spreading across the globe. Despite the rapidity in which the virus has reached a large number of countries in the world [1], transmission initially only became sustained in a subset of those countries seeded with infection from Mexico, notably the US and Southern hemisphere temperate countries. A relevant heterogeneity in the pattern of pandemic spread has been seen also within Europe: in that region, the UK has experienced a substantial first wave of transmission in the early summer, followed by a second one in the autumn, while all other European countries had only a limited transmission before the summer and a single wave in the autumn/winter [2]–[5]. Moreover, a clear West to East pattern of spread was observed for the 2009 pandemic [6], similar to that sometimes seen for seasonal flu [7]. Climatic differences (especially between northern and southern hemispheres) may be partly responsible for spatial heterogeneity in epidemic progression [8]. Human mobility patterns can also affect the spatiotemporal dynamics of an epidemic [9], [10] as well as heterogeneity in the population itself - sociodemographic structure can affect the susceptibility and contact patterns [10], [11]. For the 2009 H1N1 pandemic, the timing and length of summer school holidays [12], [13], given the emergence time, may have also affected the timing of pandemic spread in Europe. By employing an individual-based stochastic simulation model, structurally similar to those already developed for predicting the spatiotemporal spread of a flu pandemic in different geographic areas [10], [14]–[22], we analyse here which factors are most responsible for the observed geographical differences, and to which extent the pattern was predictable on the basis of the first available data on the spread of H1N1pdm in Mexico [23], the US and the UK [24]. Thus, we do not fit the model to the observed pattern of spread (which is possible only after the pandemic); rather, we use parameter values estimated from the first published analyses and examine the extent to which the model predicted spread agrees with the pattern of spread seen in the Europe in the summer and autumn of 2009. We employ extensive sensitivity analysis to assess the uncertainty in prediction, as well as the extent to which the predictions could have been improved by better parameterisation or greater detail. This allows us to also evaluate the predictability of the patterns seen and to discuss implications for the control of future pandemics. Methods Our analysis makes use of an individual-based stochastic simulation model structurally similar to a model previously developed for Europe [10]. The simulation is a spatially-explicit discrete-time SEIR model with force of infection decreasing with the geographical distance which explicitly models transmission in households, schools and workplaces. Country-specific sociodemographic data from Eurostat [25] were used to parameterise the distribution of individuals in households, schools and workplaces. Infection spread between countries is modelled through cross-border diffusion and long-distance travel, making use of European air and railway transportation data. Previous work using this model [10] did not specifically aim to model the 2009 H1N1 pandemic, but rather examined the impact of human mobility patterns and demographic heterogeneity on the expected spread of a ‘generic’ influenza pandemic, parameterised to reproduce the transmissibility of the pandemics seen in the last century. The version of the model used in that work [10] lacked some key features required to realistically capture the epidemiology of the 2009 pandemic. For this paper, we enhanced the simulation framework in a number of ways. First, rather than modelling the importation of cases through a simple compartmental model describing the spread of a pandemic outside the EU, here we explicitly model importation of cases from Mexico and US into EU countries. Since it is apparent that many features of pandemic spread in Europe depend on the timing of its emergence, the simulation in this study is synchronized to match the time of the first recorded cases across Europe. Specifically, the epidemic is seeded using country-specific data on travel-related cases in the early phase of the epidemic (up to June 3, 2009) [26]. Second, instead of assuming adults and children are equally susceptible to infection [10], [14]–[20], here we model children as being twice as susceptible to infection as adults, based on early analyses of the pandemic in Mexico [23] and the UK [24]. Third, the key role children played in the transmission of the 2009 pandemic meant that we incorporated the timing of school holidays in different EU countries, and the impact of those holidays on transmission. Fourth, the model of long-distance travel was refined to take account of the duration of stays abroad in estimating transmission risk between travellers and host populations. Last, the parameterisation of transmission rates in households, schools and workplaces was refined to match available data [12], [17], [27]. Beyond the socio-demographic information used [10], we parameterised the model using information available up to June 2009 on the generation time of the pandemic virus, T g, and on the value of the reproduction number, R 0 [23], [24]. Overall, the model has five transmission parameters: the transmission rate in households, in schools, in workplaces, in the general community and during long-distance travel. These are assumed to be identical for all European countries. For a given choice of T g, once the transmission parameters are fixed, one can estimate a value of R 0 for the model from the growth rate of the simulated epidemic. R 0 will differ between countries because of the sociodemographic differences even keeping transmission parameters constant; our reference value for comparison with data is that obtained from simulations of the pandemic in the UK, R 0 UK. We assigned the value of the five transmission parameters in such a way that R 0 UK matched early estimates of R 0 from UK data (achieved by applying an overall scaling to all transmission coefficients), and so that the proportion of transmission in different social contexts matched that estimated in past work [12], [17]: after adding the effect of age-dependent susceptibility, this results in 36% of cases being transmitted in schools, 31% in households, 9% in workplaces and 24% in the general community. During school holidays, no transmission is assumed to occur in schools, while community transmission is increased by a factor of 1.4 [12], to account for increased non-school contacts among students. The model predicts, using data on the number of nights spent by European citizens in EU countries outside their own member state, that the percentage of infections during long-distance travel is slightly lower than 0.5%. By the end of June 2009, the most reliable estimates of epidemic growth rate for the H1N1pdm pandemic were those obtained from the comprehensive (>25% population coverage) Qsurveillance sentinel surveillance system for influenza-like-illness operating in England [28]. Fitting an exponential model with non-zero intercept to data from the Qsurveillance network data available to July 1 [29] the estimated real-time exponential growth rate is 0.141/day (95% CI: 0.127–0.156), corresponding to a doubling time of 4.9 days (95% CI: 4.4–5.5 days). Such epidemic growth rates can be translated into estimates of the reproduction number, R 0, given estimates of the generation time distribution [30]. For instance, assuming exponentially distributed latent and infectious periods (as the simulation model used here does) with means of 1.5 days and 1.6 days respectively, the corresponding reproduction number estimate is 1.48 (95% CI: 1.43–1.54). Similar estimates (see supporting Text S1) are obtained using the H1N1pdm case estimates generated by the UK Health Protection Agency (HPA) which are derived from ILI data weighted by the proportion of ILI cases each week testing positive for H1N1pdm via virological surveillance, albeit the confidence bounds are wider due to the relatively small numbers of samples which were virologically tested. We therefore choose to illustrate the qualitative predictions of the model with default values of R 0 = 1.48 and T g = 3.1 days (parameters in Table 1). This choice of generation time is a little longer than the modal estimate derived from (mostly household based) contact tracing data in the early UK epidemic [24], but lies between lower and higher past estimates of the generation time of influenza [15]–[17], [31]. A sensitivity analysis is presented which demonstrates relative insensitivity of the ability of the model to predict timing of the epidemic across Europe to variation in R 0 and T g, so long as the epidemic doubling time observed in the UK in June is reproduced. 10.1371/journal.pcbi.1002205.t001 Table 1 Epidemiological parameters used in the baseline simulations (R0 = 1.48, Tg = 3.1 days). Parameter Value Transmission rate in households 0.711 days−1 Transmission rate in schools 0.840 days−1 Transmission rate in workplaces 0.408 days−1 Transmission rate in the general community 0.319 days−1 Transmission rate during long-distance travel 4.252×10−15 days−1 Transmission rate for modelling importation of cases 0.832 days−1 Latent period 1.5 days Infectious period 1.6 days Relative susceptibility to infection of adults with respect to children 0.5 Details on the model structure and its parameterisation are given in the supporting Text S1. Results Most trips from the US and Mexico to the EU are to Western European countries and especially to the UK (about one third of the trips, see supporting Text S1). We found that the date of the first case in each European country [32] correlates significantly with the number of travellers to the country from Mexico and US, both in the data and in model output (see Table 2) and with longitude (a West to East pattern is observed; see Table 2). 10.1371/journal.pcbi.1002205.t002 Table 2 Correlation of population variables and epidemic statistics as observed or predicted by the model in the different European countries. Epidemic statistic Population variable Correlation as observed in the data Correlation as predicted by the model Day of the first caseh US-MX travellers a ρ = −0.875 p 30). Light green indicates 0.5