- Record: found
- Abstract: found
- Article: found
Wnt ligands from the embryonic surface ectoderm regulate ‘bimetallic strip’ optic cup morphogenesis in mouse
Read this article at
Abstract
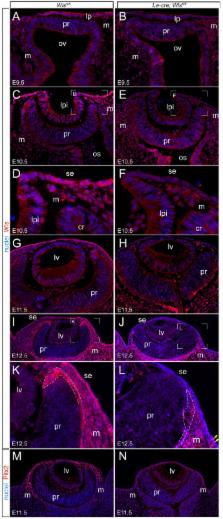
The Wnt/β-catenin response pathway is central to many developmental processes. Here, we assessed the role of Wnt signaling in early eye development using the mouse as a model system. We showed that the surface ectoderm region that includes the lens placode expressed 12 out of 19 possible Wnt ligands. When these activities were suppressed by conditional deletion of wntless ( Le-cre; Wls fl/fl ) there were dramatic consequences that included a saucer-shaped optic cup, ventral coloboma, and a deficiency of periocular mesenchyme. This phenotype shared features with that produced when the Wnt/β-catenin pathway co-receptor Lrp6 is mutated or when retinoic acid (RA) signaling in the eye is compromised. Consistent with this, microarray and cell fate marker analysis identified a series of expression changes in genes known to be regulated by RA or by the Wnt/β-catenin pathway. Using pathway reporters, we showed that Wnt ligands from the surface ectoderm directly or indirectly elicit a Wnt/β-catenin response in retinal pigment epithelium (RPE) progenitors near the optic cup rim. In Le-cre; Wls fl/fl mice, the numbers of RPE cells are reduced and this can explain, using the principle of the bimetallic strip, the curvature of the optic cup. These data thus establish a novel hypothesis to explain how differential cell numbers in a bilayered epithelium can lead to shape change.
Abstract
Summary: During optic cup morphogenesis, Wnt ligands expressed in the surface ectoderm control cell proliferation in the retinal pigmented epithelium, and thus influence bending of the neural retina.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation.
- Record: found
- Abstract: found
- Article: not found
Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision.
- Record: found
- Abstract: found
- Article: not found